Ian Pan
Best Practices and Scoring System on Reviewing A.I. based Medical Imaging Papers: Part 1 Classification
Feb 03, 2022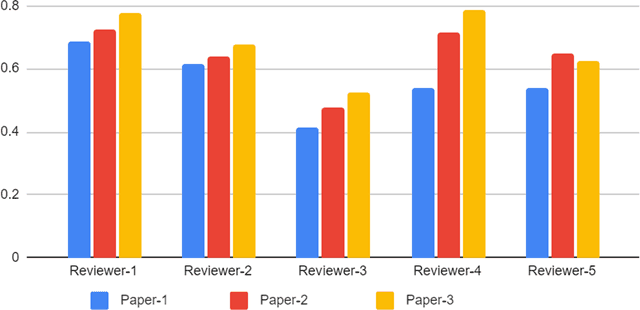
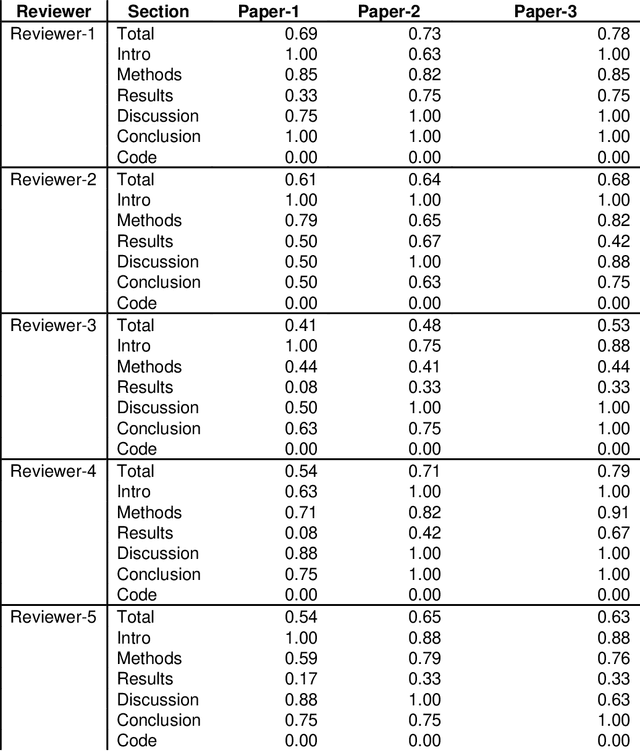
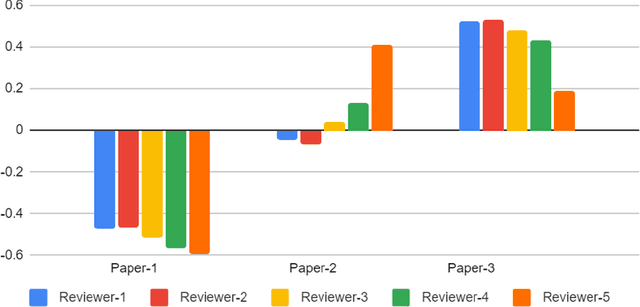
Abstract:With the recent advances in A.I. methodologies and their application to medical imaging, there has been an explosion of related research programs utilizing these techniques to produce state-of-the-art classification performance. Ultimately, these research programs culminate in submission of their work for consideration in peer reviewed journals. To date, the criteria for acceptance vs. rejection is often subjective; however, reproducible science requires reproducible review. The Machine Learning Education Sub-Committee of SIIM has identified a knowledge gap and a serious need to establish guidelines for reviewing these studies. Although there have been several recent papers with this goal, this present work is written from the machine learning practitioners standpoint. In this series, the committee will address the best practices to be followed in an A.I.-based study and present the required sections in terms of examples and discussion of what should be included to make the studies cohesive, reproducible, accurate, and self-contained. This first entry in the series focuses on the task of image classification. Elements such as dataset curation, data pre-processing steps, defining an appropriate reference standard, data partitioning, model architecture and training are discussed. The sections are presented as they would be detailed in a typical manuscript, with content describing the necessary information that should be included to make sure the study is of sufficient quality to be considered for publication. The goal of this series is to provide resources to not only help improve the review process for A.I.-based medical imaging papers, but to facilitate a standard for the information that is presented within all components of the research study. We hope to provide quantitative metrics in what otherwise may be a qualitative review process.
A comparative study of 2D image segmentation algorithms for traumatic brain lesions using CT data from the ProTECTIII multicenter clinical trial
Jun 01, 2020Abstract:Automated segmentation of medical imaging is of broad interest to clinicians and machine learning researchers alike. The goal of segmentation is to increase efficiency and simplicity of visualization and quantification of regions of interest within a medical image. Image segmentation is a difficult task because of multiparametric heterogeneity within the images, an obstacle that has proven especially challenging in efforts to automate the segmentation of brain lesions from non-contrast head computed tomography (CT). In this research, we have experimented with multiple available deep learning architectures to segment different phenotypes of hemorrhagic lesions found after moderate to severe traumatic brain injury (TBI). These include: intraparenchymal hemorrhage (IPH), subdural hematoma (SDH), epidural hematoma (EDH), and traumatic contusions. We were able to achieve an optimal Dice Coefficient1 score of 0.94 using UNet++ 2D Architecture with Focal Tversky Loss Function, an increase from 0.85 using UNet 2D with Binary Cross-Entropy Loss Function in intraparenchymal hemorrhage (IPH) cases. Furthermore, using the same setting, we were able to achieve the Dice Coefficient score of 0.90 and 0.86 in cases of Extra-Axial bleeds and Traumatic contusions, respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge