Hagen Wenzek
Time-based Self-supervised Learning for Wireless Capsule Endoscopy
Apr 20, 2022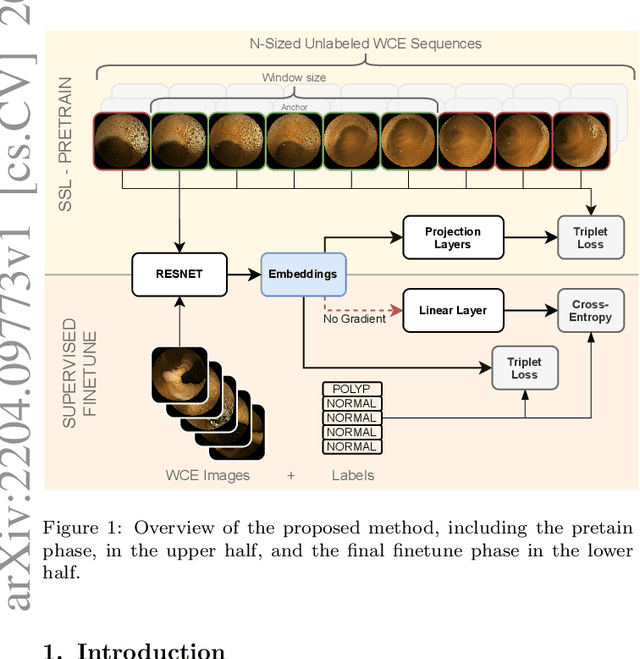
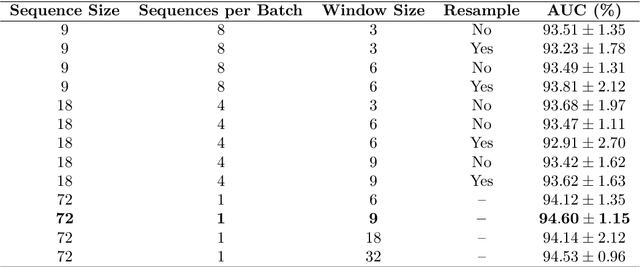
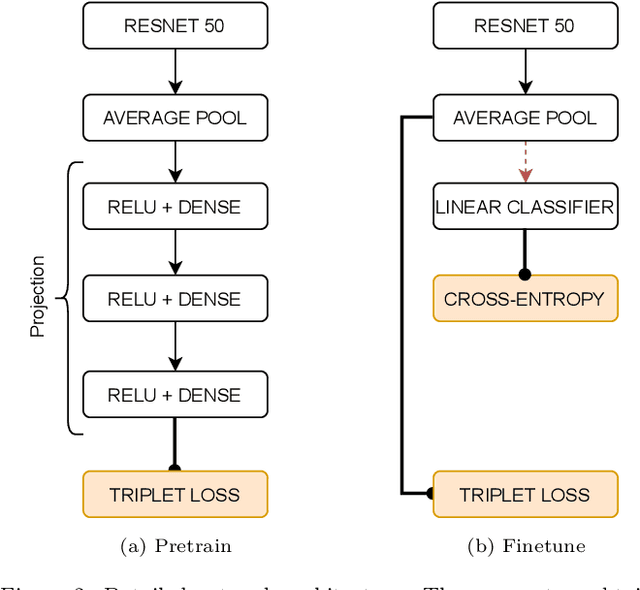
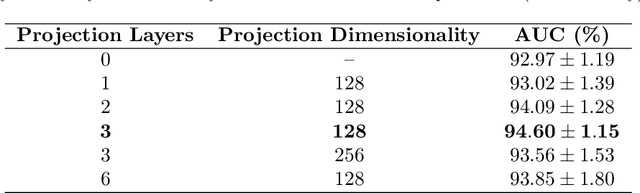
Abstract:State-of-the-art machine learning models, and especially deep learning ones, are significantly data-hungry; they require vast amounts of manually labeled samples to function correctly. However, in most medical imaging fields, obtaining said data can be challenging. Not only the volume of data is a problem, but also the imbalances within its classes; it is common to have many more images of healthy patients than of those with pathology. Computer-aided diagnostic systems suffer from these issues, usually over-designing their models to perform accurately. This work proposes using self-supervised learning for wireless endoscopy videos by introducing a custom-tailored method that does not initially need labels or appropriate balance. We prove that using the inferred inherent structure learned by our method, extracted from the temporal axis, improves the detection rate on several domain-specific applications even under severe imbalance.
WCE Polyp Detection with Triplet based Embeddings
Dec 10, 2019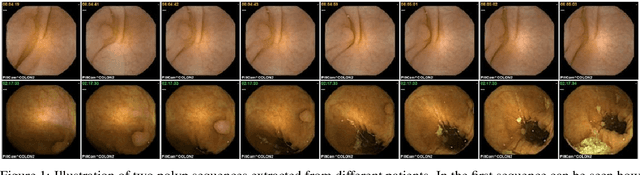
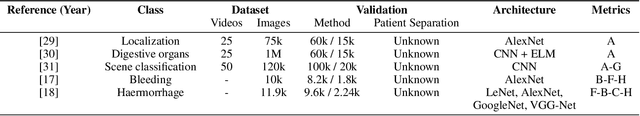

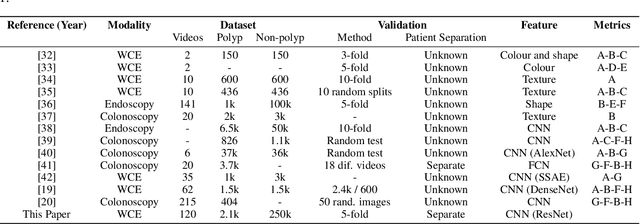
Abstract:Wireless capsule endoscopy is a medical procedure used to visualize the entire gastrointestinal tract and to diagnose intestinal conditions, such as polyps or bleeding. Current analyses are performed by manually inspecting nearly each one of the frames of the video, a tedious and error-prone task. Automatic image analysis methods can be used to reduce the time needed for physicians to evaluate a capsule endoscopy video, however these methods are still in a research phase. In this paper we focus on computer-aided polyp detection in capsule endoscopy images. This is a challenging problem because of the diversity of polyp appearance, the imbalanced dataset structure and the scarcity of data. We have developed a new polyp computer-aided decision system that combines a deep convolutional neural network and metric learning. The key point of the method is the use of the triplet loss function with the aim of improving feature extraction from the images when having small dataset. The triplet loss function allows to train robust detectors by forcing images from the same category to be represented by similar embedding vectors while ensuring that images from different categories are represented by dissimilar vectors. Empirical results show a meaningful increase of AUC values compared to baseline methods. A good performance is not the only requirement when considering the adoption of this technology to clinical practice. Trust and explainability of decisions are as important as performance. With this purpose, we also provide a method to generate visual explanations of the outcome of our polyp detector. These explanations can be used to build a physician's trust in the system and also to convey information about the inner working of the method to the designer for debugging purposes.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge