Grzegorz Mrukwa
Quantitative Impact of Label Noise on the Quality of Segmentation of Brain Tumors on MRI scans
Sep 18, 2019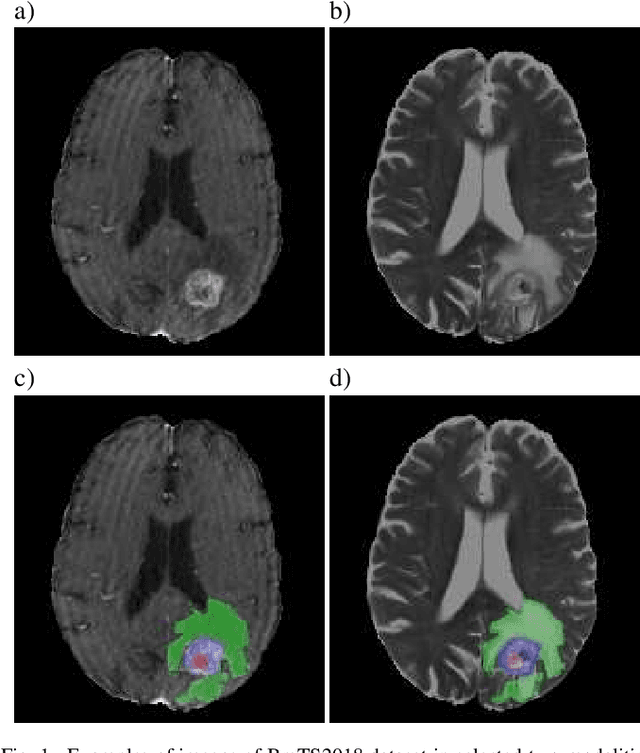
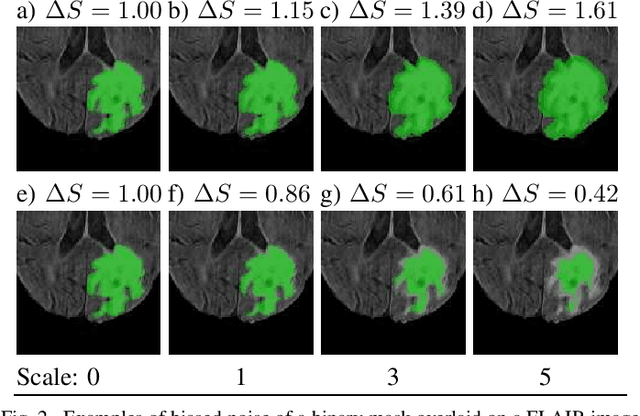
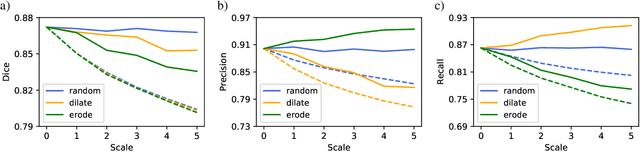
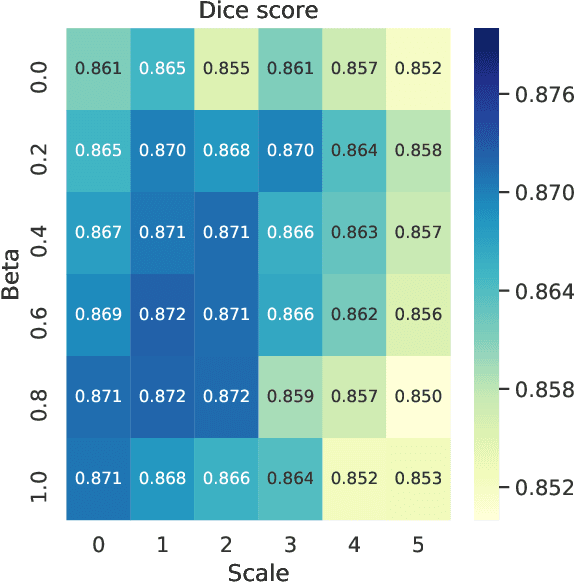
Abstract:Over the last few years, deep learning has proven to be a great solution to many problems, such as image or text classification. Recently, deep learning-based solutions have outperformed humans on selected benchmark datasets, yielding a promising future for scientific and real-world applications. Training of deep learning models requires vast amounts of high quality data to achieve such supreme performance. In real-world scenarios, obtaining a large, coherent, and properly labeled dataset is a challenging task. This is especially true in medical applications, where high-quality data and annotations are scarce and the number of expert annotators is limited. In this paper, we investigate the impact of corrupted ground-truth masks on the performance of a neural network for a brain tumor segmentation task. Our findings suggest that a) the performance degrades about 8% less than it could be expected from simulations, b) a neural network learns the simulated biases of annotators, c) biases can be partially mitigated by using an inversely-biased dice loss function.
Fully-automated deep learning-powered system for DCE-MRI analysis of brain tumors
Jul 18, 2019



Abstract:Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) plays an important role in diagnosis and grading of brain tumor. Although manual DCE biomarker extraction algorithms boost the diagnostic yield of DCE-MRI by providing quantitative information on tumor prognosis and prediction, they are time-consuming and prone to human error. In this paper, we propose a fully-automated, end-to-end system for DCE-MRI analysis of brain tumors. Our deep learning-powered technique does not require any user interaction, it yields reproducible results, and it is rigorously validated against benchmark (BraTS'17 for tumor segmentation, and a test dataset released by the Quantitative Imaging Biomarkers Alliance for the contrast-concentration fitting) and clinical (44 low-grade glioma patients) data. Also, we introduce a cubic model of the vascular input function used for pharmacokinetic modeling which significantly decreases the fitting error when compared with the state of the art, alongside a real-time algorithm for determination of the vascular input region. An extensive experimental study, backed up with statistical tests, showed that our system delivers state-of-the-art results (in terms of segmentation accuracy and contrast-concentration fitting) while requiring less than 3 minutes to process an entire input DCE-MRI study using a single GPU.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge