Gabrielle Phillips
FDNet: Frequency Domain Denoising Network For Cell Segmentation in Astrocytes Derived From Induced Pluripotent Stem Cells
Feb 05, 2024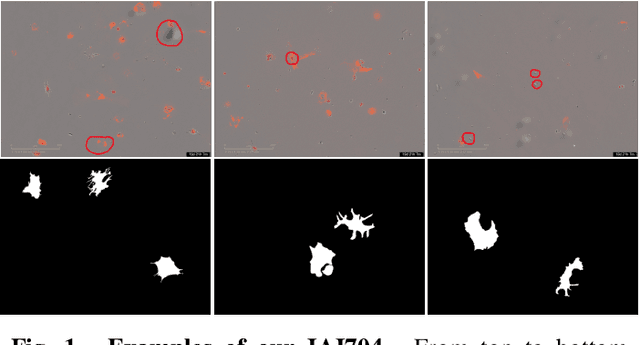
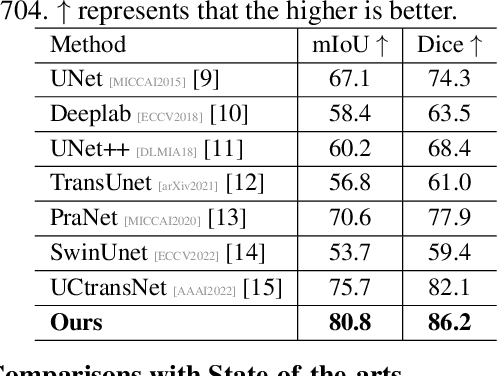
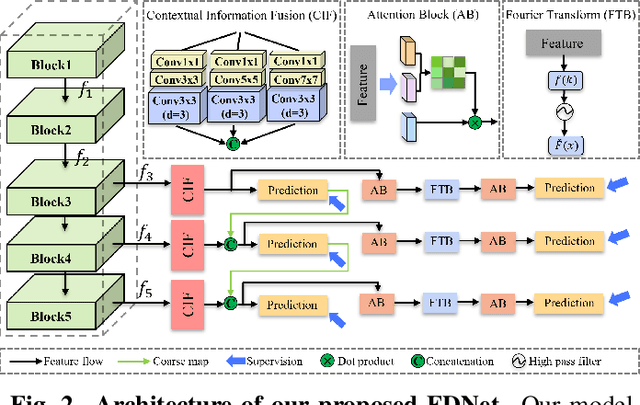
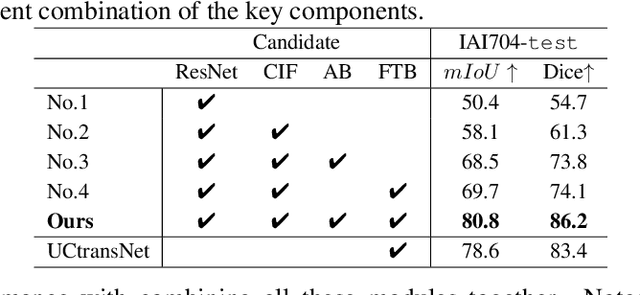
Abstract:Artificially generated induced pluripotent stem cells (iPSCs) from somatic cells play an important role for disease modeling and drug screening of neurodegenerative diseases. Astrocytes differentiated from iPSCs are important targets to investigate neuronal metabolism. The astrocyte differentiation progress can be monitored through the variations of morphology observed from microscopy images at different differentiation stages, then determined by molecular biology techniques upon maturation. However, the astrocytes usually ``perfectly'' blend into the background and some of them are covered by interference information (i.e., dead cells, media sediments, and cell debris), which makes astrocytes difficult to observe. Due to the lack of annotated datasets, the existing state-of-the-art deep learning approaches cannot be used to address this issue. In this paper, we introduce a new task named astrocyte segmentation with a novel dataset, called IAI704, which contains 704 images and their corresponding pixel-level annotation masks. Moreover, a novel frequency domain denoising network, named FDNet, is proposed for astrocyte segmentation. In detail, our FDNet consists of a contextual information fusion module (CIF), an attention block (AB), and a Fourier transform block (FTB). CIF and AB fuse multi-scale feature embeddings to localize the astrocytes. FTB transforms feature embeddings into the frequency domain and conducts a high-pass filter to eliminate interference information. Experimental results demonstrate the superiority of our proposed FDNet over the state-of-the-art substitutes in astrocyte segmentation, shedding insights for iPSC differentiation progress prediction.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge