Filip Miljković
Mask prior-guided denoising diffusion improves inverse protein folding
Dec 10, 2024



Abstract:Inverse protein folding generates valid amino acid sequences that can fold into a desired protein structure, with recent deep-learning advances showing significant potential and competitive performance. However, challenges remain in predicting highly uncertain regions, such as those with loops and disorders. To tackle such low-confidence residue prediction, we propose a \textbf{Ma}sk \textbf{p}rior-guided denoising \textbf{Diff}usion (\textbf{MapDiff}) framework that accurately captures both structural and residue interactions for inverse protein folding. MapDiff is a discrete diffusion probabilistic model that iteratively generates amino acid sequences with reduced noise, conditioned on a given protein backbone. To incorporate structural and residue interactions, we develop a graph-based denoising network with a mask prior pre-training strategy. Moreover, in the generative process, we combine the denoising diffusion implicit model with Monte-Carlo dropout to improve uncertainty estimation. Evaluation on four challenging sequence design benchmarks shows that MapDiff significantly outperforms state-of-the-art methods. Furthermore, the in-silico sequences generated by MapDiff closely resemble the physico-chemical and structural characteristics of native proteins across different protein families and architectures.
Interpretable bilinear attention network with domain adaptation improves drug-target prediction
Aug 03, 2022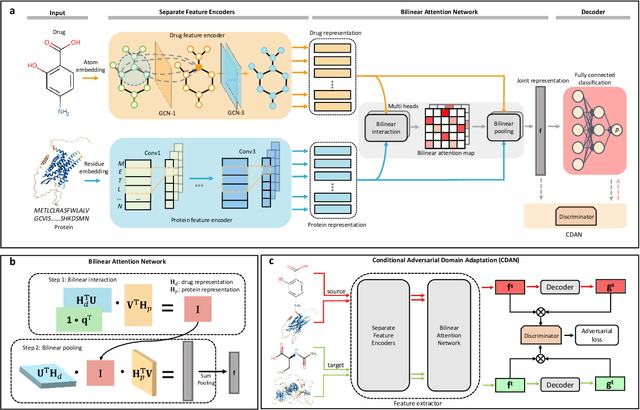
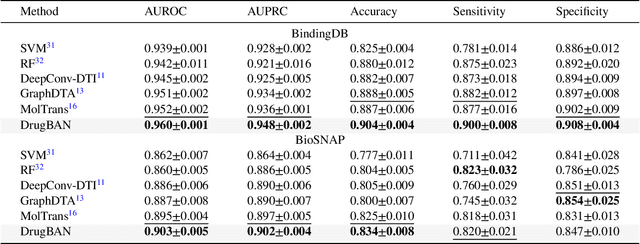


Abstract:Predicting drug-target interaction is key for drug discovery. Recent deep learning-based methods show promising performance but two challenges remain: (i) how to explicitly model and learn local interactions between drugs and targets for better prediction and interpretation; (ii) how to generalize prediction performance on novel drug-target pairs from different distribution. In this work, we propose DrugBAN, a deep bilinear attention network (BAN) framework with domain adaptation to explicitly learn pair-wise local interactions between drugs and targets, and adapt on out-of-distribution data. DrugBAN works on drug molecular graphs and target protein sequences to perform prediction, with conditional domain adversarial learning to align learned interaction representations across different distributions for better generalization on novel drug-target pairs. Experiments on three benchmark datasets under both in-domain and cross-domain settings show that DrugBAN achieves the best overall performance against five state-of-the-art baselines. Moreover, visualizing the learned bilinear attention map provides interpretable insights from prediction results.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge