Fanglin Linda Liu
Dynamic Structured Illumination Microscopy with a Neural Space-time Model
Jun 03, 2022
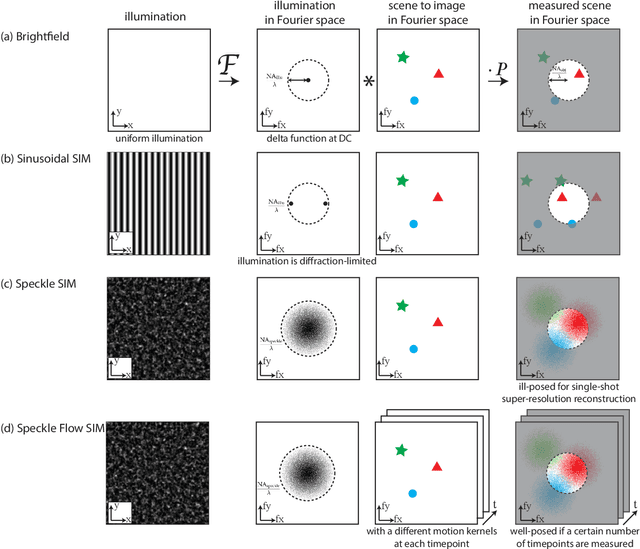


Abstract:Structured illumination microscopy (SIM) reconstructs a super-resolved image from multiple raw images; hence, acquisition speed is limited, making it unsuitable for dynamic scenes. We propose a new method, Speckle Flow SIM, that models sample motion during the data capture in order to reconstruct dynamic scenes with super-resolution. Speckle Flow SIM uses fixed speckle illumination and relies on sample motion to capture a sequence of raw images. Then, the spatio-temporal relationship of the dynamic scene is modeled using a neural space-time model with coordinate-based multi-layer perceptrons (MLPs), and the motion dynamics and the super-resolved scene are jointly recovered. We validated Speckle Flow SIM in simulation and built a simple, inexpensive experimental setup with off-the-shelf components. We demonstrated that Speckle Flow SIM can reconstruct a dynamic scene with deformable motion and 1.88x the diffraction-limited resolution in experiment.
Miniscope3D: optimized single-shot miniature 3D fluorescence microscopy
Oct 12, 2020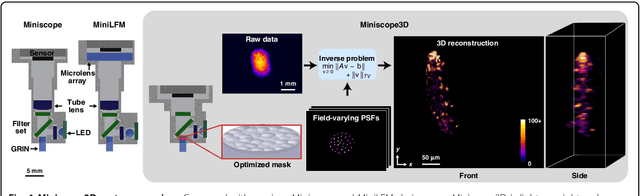
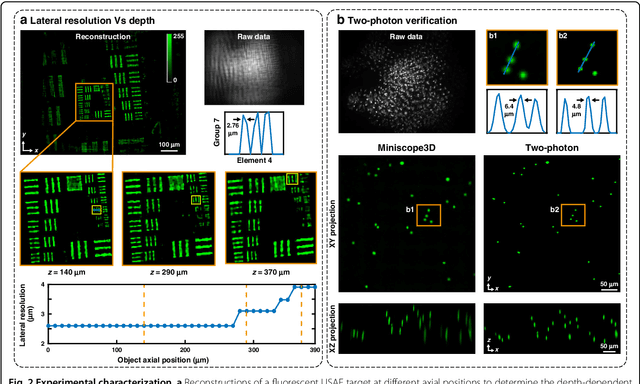
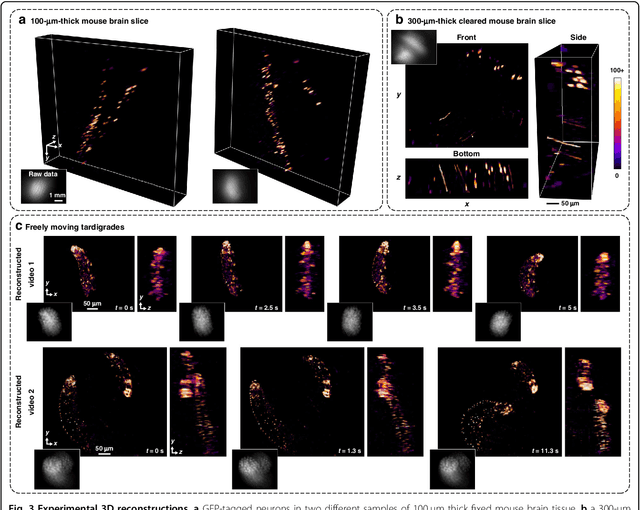

Abstract:Miniature fluorescence microscopes are a standard tool in systems biology. However, widefield miniature microscopes capture only 2D information, and modifications that enable 3D capabilities increase the size and weight and have poor resolution outside a narrow depth range. Here, we achieve the 3D capability by replacing the tube lens of a conventional 2D Miniscope with an optimized multifocal phase mask at the objective's aperture stop. Placing the phase mask at the aperture stop significantly reduces the size of the device, and varying the focal lengths enables a uniform resolution across a wide depth range. The phase mask encodes the 3D fluorescence intensity into a single 2D measurement, and the 3D volume is recovered by solving a sparsity-constrained inverse problem. We provide methods for designing and fabricating the phase mask and an efficient forward model that accounts for the field-varying aberrations in miniature objectives. We demonstrate a prototype that is 17 mm tall and weighs 2.5 grams, achieving 2.76 $\mu$m lateral, and 15 $\mu$m axial resolution across most of the 900x700x390 $\mu m^3$ volume at 40 volumes per second. The performance is validated experimentally on resolution targets, dynamic biological samples, and mouse brain tissue. Compared with existing miniature single-shot volume-capture implementations, our system is smaller and lighter and achieves a more than 2x better lateral and axial resolution throughout a 10x larger usable depth range. Our microscope design provides single-shot 3D imaging for applications where a compact platform matters, such as volumetric neural imaging in freely moving animals and 3D motion studies of dynamic samples in incubators and lab-on-a-chip devices.
* Published with Nature Springer in Light: Science and Applications
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge