Faeze Gholamiankhah
Automated lung segmentation from CT images of normal and COVID-19 pneumonia patients
Apr 05, 2021
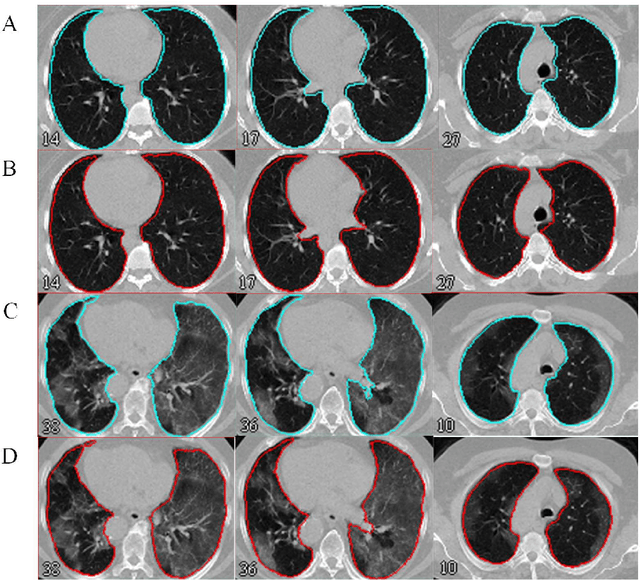
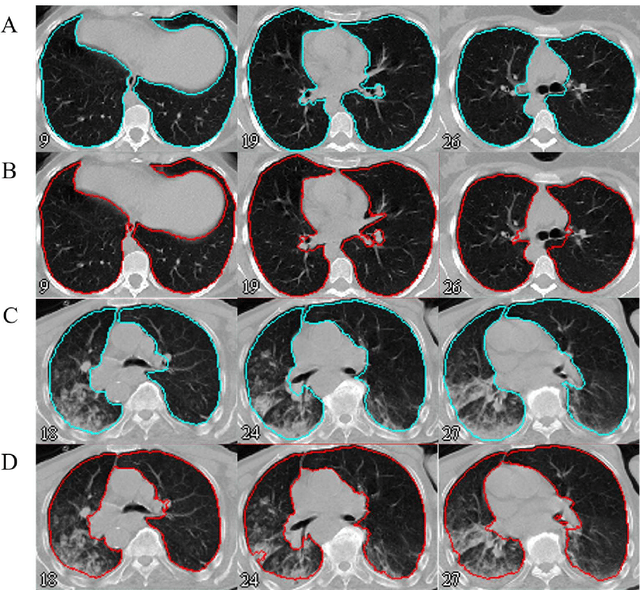
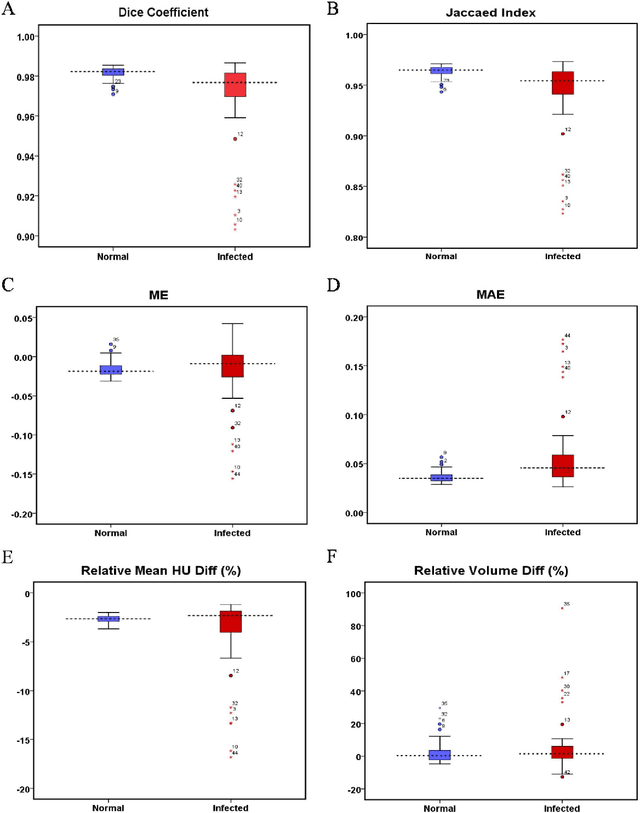
Abstract:Automated semantic image segmentation is an essential step in quantitative image analysis and disease diagnosis. This study investigates the performance of a deep learning-based model for lung segmentation from CT images for normal and COVID-19 patients. Chest CT images and corresponding lung masks of 1200 confirmed COVID-19 cases were used for training a residual neural network. The reference lung masks were generated through semi-automated/manual segmentation of the CT images. The performance of the model was evaluated on two distinct external test datasets including 120 normal and COVID-19 subjects, and the results of these groups were compared to each other. Different evaluation metrics such as dice coefficient (DSC), mean absolute error (MAE), relative mean HU difference, and relative volume difference were calculated to assess the accuracy of the predicted lung masks. The proposed deep learning method achieved DSC of 0.980 and 0.971 for normal and COVID-19 subjects, respectively, demonstrating significant overlap between predicted and reference lung masks. Moreover, MAEs of 0.037 HU and 0.061 HU, relative mean HU difference of -2.679% and -4.403%, and relative volume difference of 2.405% and 5.928% were obtained for normal and COVID-19 subjects, respectively. The comparable performance in lung segmentation of the normal and COVID-19 patients indicates the accuracy of the model for the identification of the lung tissue in the presence of the COVID-19 induced infections (though slightly better performance was observed for normal patients). The promising results achieved by the proposed deep learning-based model demonstrated its reliability in COVID-19 lung segmentation. This prerequisite step would lead to a more efficient and robust pneumonia lesion analysis.
Deep learning-based synthetic CT generation from MR images: comparison of generative adversarial and residual neural networks
Mar 02, 2021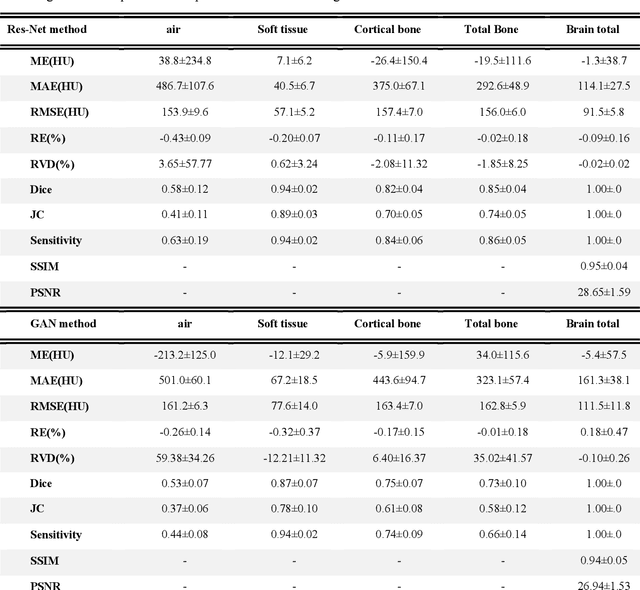
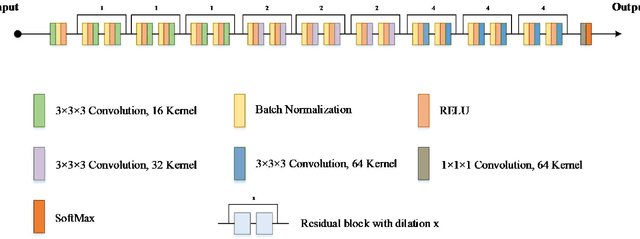
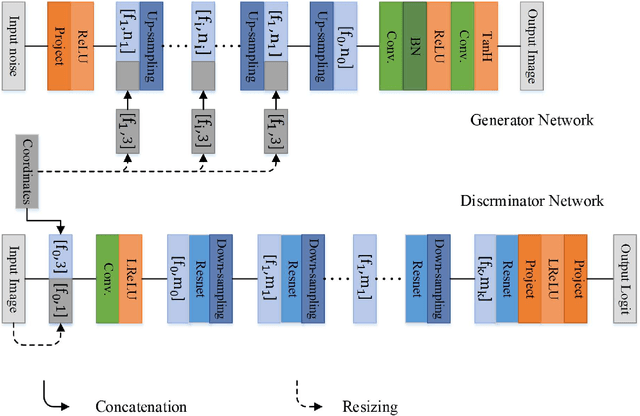
Abstract:Currently, MRI-only radiotherapy (RT) eliminates some of the concerns about using CT images in RT chains such as the registration of MR images to a separate CT, extra dose delivery, and the additional cost of repeated imaging. However, one remaining challenge is that the signal intensities of MRI are not related to the attenuation coefficient of the biological tissue. This work compares the performance of two state-of-the-art deep learning models; a generative adversarial network (GAN) and a residual network (ResNet) for synthetic CTs (sCT) generation from MR images. The brain MR and CT images of 86 participants were analyzed. GAN and ResNet models were implemented for the generation of synthetic CTs from the 3D T1-weighted MR images using a six-fold cross-validation scheme. The resulting sCTs were compared, considering the CT images as a reference using standard metrics such as the mean absolute error (MAE), peak signal-to-noise ratio (PSNR) and the structural similarity index (SSIM). Overall, the ResNet model exhibited higher accuracy in relation to the delineation of brain tissues. The ResNet model estimated the CT values for the entire head region with an MAE of 114.1 HU compared to MAE=-10.9 HU obtained from the GAN model. Moreover, both models offered comparable SSIM and PSNR values, although the ResNet method exhibited a slightly superior performance over the GAN method. We compared two state-of-the-art deep learning models for the task of MR-based sCT generation. The ResNet model exhibited superior results, thus demonstrating its potential to be used for the challenge of synthetic CT generation in PET/MR AC and MR-only RT planning.
Deep learning-based attenuation correction in the image domain for myocardial perfusion SPECT imaging
Feb 10, 2021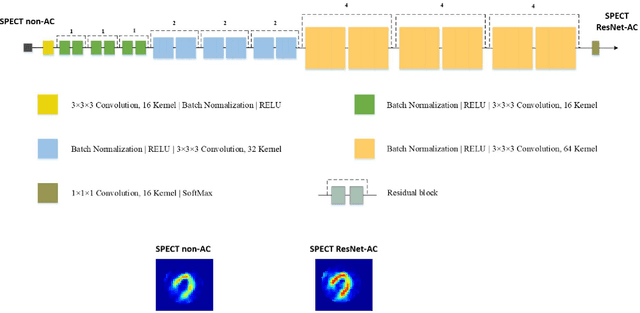
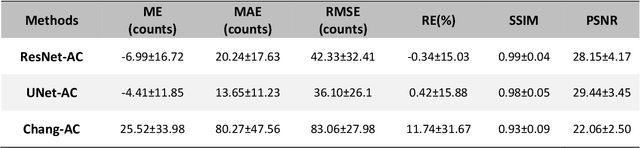
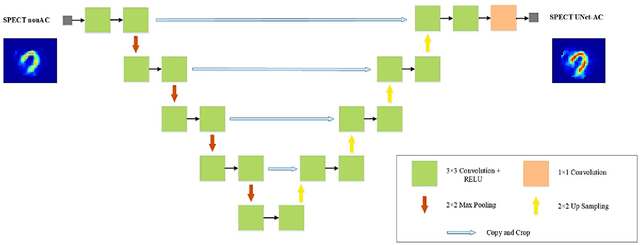
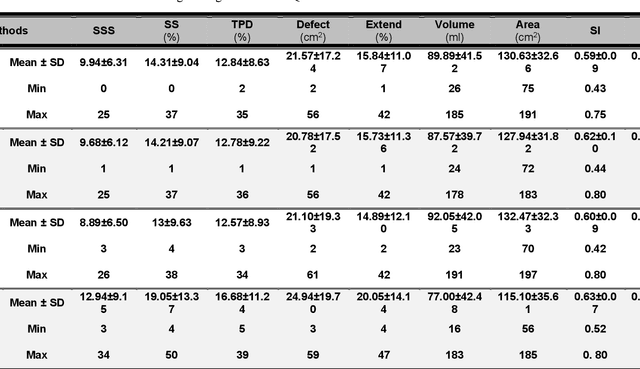
Abstract:Objective: In this work, we set out to investigate the accuracy of direct attenuation correction (AC) in the image domain for the myocardial perfusion SPECT imaging (MPI-SPECT) using two residual (ResNet) and UNet deep convolutional neural networks. Methods: The MPI-SPECT 99mTc-sestamibi images of 99 participants were retrospectively examined. UNet and ResNet networks were trained using SPECT non-attenuation corrected images as input and CT-based attenuation corrected SPECT images (CT-AC) as reference. The Chang AC approach, considering a uniform attenuation coefficient within the body contour, was also implemented. Quantitative and clinical evaluation of the proposed methods were performed considering SPECT CT-AC images of 19 subjects as reference using the mean absolute error (MAE), structural similarity index (SSIM) metrics, as well as relevant clinical indices such as perfusion deficit (TPD). Results: Overall, the deep learning solution exhibited good agreement with the CT-based AC, noticeably outperforming the Chang method. The ResNet and UNet models resulted in the ME (count) of ${-6.99\pm16.72}$ and ${-4.41\pm11.8}$ and SSIM of ${0.99\pm0.04}$ and ${0.98\pm0.05}$, respectively. While the Change approach led to ME and SSIM of ${25.52\pm33.98}$ and ${0.93\pm0.09}$, respectively. Similarly, the clinical evaluation revealed a mean TPD of ${12.78\pm9.22}$ and ${12.57\pm8.93}$ for the ResNet and UNet models, respectively, compared to ${12.84\pm8.63}$ obtained from the reference SPECT CT-AC images. On the other hand, the Chang approach led to a mean TPD of ${16.68\pm11.24}$. Conclusion: We evaluated two deep convolutional neural networks to estimate SPECT-AC images directly from the non-attenuation corrected images. The deep learning solutions exhibited the promising potential to generate reliable attenuation corrected SPECT images without the use of transmission scanning.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge