Difei Gu
LUCID-SAE: Learning Unified Vision-Language Sparse Codes for Interpretable Concept Discovery
Feb 07, 2026Abstract:Sparse autoencoders (SAEs) offer a natural path toward comparable explanations across different representation spaces. However, current SAEs are trained per modality, producing dictionaries whose features are not directly understandable and whose explanations do not transfer across domains. In this study, we introduce LUCID (Learning Unified vision-language sparse Codes for Interpretable concept Discovery), a unified vision-language sparse autoencoder that learns a shared latent dictionary for image patch and text token representations, while reserving private capacity for modality-specific details. We achieve feature alignment by coupling the shared codes with a learned optimal transport matching objective without the need of labeling. LUCID yields interpretable shared features that support patch-level grounding, establish cross-modal neuron correspondence, and enhance robustness against the concept clustering problem in similarity-based evaluation. Leveraging the alignment properties, we develop an automated dictionary interpretation pipeline based on term clustering without manual observations. Our analysis reveals that LUCID's shared features capture diverse semantic categories beyond objects, including actions, attributes, and abstract concepts, demonstrating a comprehensive approach to interpretable multimodal representations.
Anatomy-VLM: A Fine-grained Vision-Language Model for Medical Interpretation
Nov 11, 2025Abstract:Accurate disease interpretation from radiology remains challenging due to imaging heterogeneity. Achieving expert-level diagnostic decisions requires integration of subtle image features with clinical knowledge. Yet major vision-language models (VLMs) treat images as holistic entities and overlook fine-grained image details that are vital for disease diagnosis. Clinicians analyze images by utilizing their prior medical knowledge and identify anatomical structures as important region of interests (ROIs). Inspired from this human-centric workflow, we introduce Anatomy-VLM, a fine-grained, vision-language model that incorporates multi-scale information. First, we design a model encoder to localize key anatomical features from entire medical images. Second, these regions are enriched with structured knowledge for contextually-aware interpretation. Finally, the model encoder aligns multi-scale medical information to generate clinically-interpretable disease prediction. Anatomy-VLM achieves outstanding performance on both in- and out-of-distribution datasets. We also validate the performance of Anatomy-VLM on downstream image segmentation tasks, suggesting that its fine-grained alignment captures anatomical and pathology-related knowledge. Furthermore, the Anatomy-VLM's encoder facilitates zero-shot anatomy-wise interpretation, providing its strong expert-level clinical interpretation capabilities.
RadAlign: Advancing Radiology Report Generation with Vision-Language Concept Alignment
Jan 13, 2025Abstract:Automated chest radiographs interpretation requires both accurate disease classification and detailed radiology report generation, presenting a significant challenge in the clinical workflow. Current approaches either focus on classification accuracy at the expense of interpretability or generate detailed but potentially unreliable reports through image captioning techniques. In this study, we present RadAlign, a novel framework that combines the predictive accuracy of vision-language models (VLMs) with the reasoning capabilities of large language models (LLMs). Inspired by the radiologist's workflow, RadAlign first employs a specialized VLM to align visual features with key medical concepts, achieving superior disease classification with an average AUC of 0.885 across multiple diseases. These recognized medical conditions, represented as text-based concepts in the aligned visual-language space, are then used to prompt LLM-based report generation. Enhanced by a retrieval-augmented generation mechanism that grounds outputs in similar historical cases, RadAlign delivers superior report quality with a GREEN score of 0.678, outperforming state-of-the-art methods' 0.634. Our framework maintains strong clinical interpretability while reducing hallucinations, advancing automated medical imaging and report analysis through integrated predictive and generative AI. Code is available at https://github.com/difeigu/RadAlign.
Aligning Human Knowledge with Visual Concepts Towards Explainable Medical Image Classification
Jun 08, 2024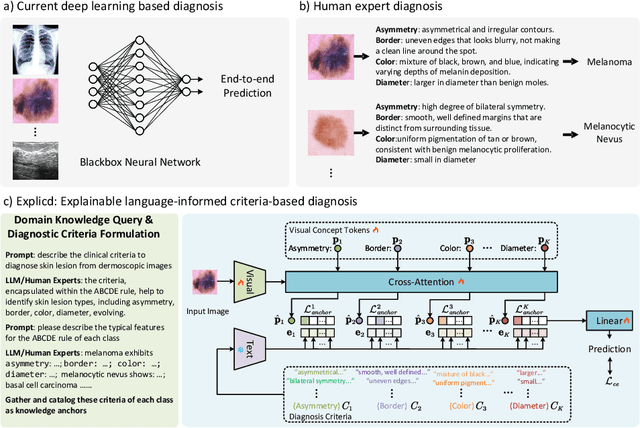
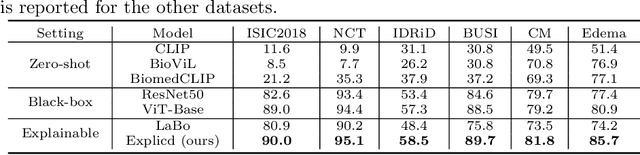
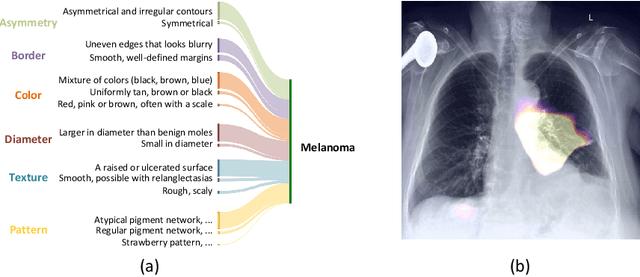
Abstract:Although explainability is essential in the clinical diagnosis, most deep learning models still function as black boxes without elucidating their decision-making process. In this study, we investigate the explainable model development that can mimic the decision-making process of human experts by fusing the domain knowledge of explicit diagnostic criteria. We introduce a simple yet effective framework, Explicd, towards Explainable language-informed criteria-based diagnosis. Explicd initiates its process by querying domain knowledge from either large language models (LLMs) or human experts to establish diagnostic criteria across various concept axes (e.g., color, shape, texture, or specific patterns of diseases). By leveraging a pretrained vision-language model, Explicd injects these criteria into the embedding space as knowledge anchors, thereby facilitating the learning of corresponding visual concepts within medical images. The final diagnostic outcome is determined based on the similarity scores between the encoded visual concepts and the textual criteria embeddings. Through extensive evaluation of five medical image classification benchmarks, Explicd has demonstrated its inherent explainability and extends to improve classification performance compared to traditional black-box models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge