Dietrich Kohlheyer
EAP4EMSIG -- Enhancing Event-Driven Microscopy for Microfluidic Single-Cell Analysis
Mar 30, 2025Abstract:Microfluidic Live-Cell Imaging yields data on microbial cell factories. However, continuous acquisition is challenging as high-throughput experiments often lack realtime insights, delaying responses to stochastic events. We introduce three components in the Experiment Automation Pipeline for Event-Driven Microscopy to Smart Microfluidic Single-Cell Analysis: a fast, accurate Deep Learning autofocusing method predicting the focus offset, an evaluation of real-time segmentation methods and a realtime data analysis dashboard. Our autofocusing achieves a Mean Absolute Error of 0.0226\textmu m with inference times below 50~ms. Among eleven Deep Learning segmentation methods, Cellpose~3 reached a Panoptic Quality of 93.58\%, while a distance-based method is fastest (121~ms, Panoptic Quality 93.02\%). All six Deep Learning Foundation Models were unsuitable for real-time segmentation.
EAP4EMSIG -- Experiment Automation Pipeline for Event-Driven Microscopy to Smart Microfluidic Single-Cells Analysis
Nov 06, 2024Abstract:Microfluidic Live-Cell Imaging (MLCI) generates high-quality data that allows biotechnologists to study cellular growth dynamics in detail. However, obtaining these continuous data over extended periods is challenging, particularly in achieving accurate and consistent real-time event classification at the intersection of imaging and stochastic biology. To address this issue, we introduce the Experiment Automation Pipeline for Event-Driven Microscopy to Smart Microfluidic Single-Cells Analysis (EAP4EMSIG). In particular, we present initial zero-shot results from the real-time segmentation module of our approach. Our findings indicate that among four State-Of-The- Art (SOTA) segmentation methods evaluated, Omnipose delivers the highest Panoptic Quality (PQ) score of 0.9336, while Contour Proposal Network (CPN) achieves the fastest inference time of 185 ms with the second-highest PQ score of 0.8575. Furthermore, we observed that the vision foundation model Segment Anything is unsuitable for this particular use case.
Cell tracking for live-cell microscopy using an activity-prioritized assignment strategy
Oct 20, 2022
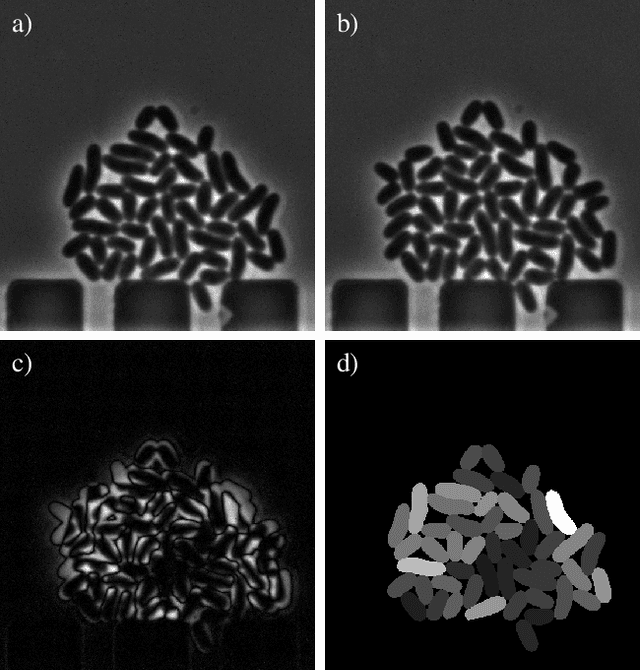
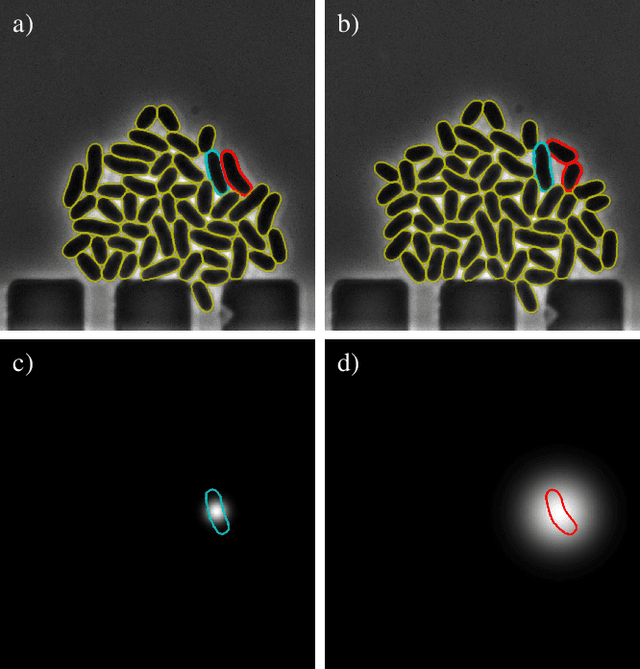
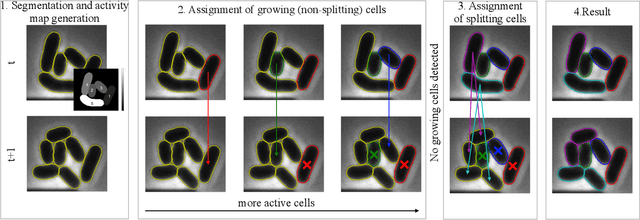
Abstract:Cell tracking is an essential tool in live-cell imaging to determine single-cell features, such as division patterns or elongation rates. Unlike in common multiple object tracking, in microbial live-cell experiments cells are growing, moving, and dividing over time, to form cell colonies that are densely packed in mono-layer structures. With increasing cell numbers, following the precise cell-cell associations correctly over many generations becomes more and more challenging, due to the massively increasing number of possible associations. To tackle this challenge, we propose a fast parameter-free cell tracking approach, which consists of activity-prioritized nearest neighbor assignment of growing cells and a combinatorial solver that assigns splitting mother cells to their daughters. As input for the tracking, Omnipose is utilized for instance segmentation. Unlike conventional nearest-neighbor-based tracking approaches, the assignment steps of our proposed method are based on a Gaussian activity-based metric, predicting the cell-specific migration probability, thereby limiting the number of erroneous assignments. In addition to being a building block for cell tracking, the proposed activity map is a standalone tracking-free metric for indicating cell activity. Finally, we perform a quantitative analysis of the tracking accuracy for different frame rates, to inform life scientists about a suitable (in terms of tracking performance) choice of the frame rate for their cultivation experiments, when cell tracks are the desired key outcome.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge