Danny Barash
Leveraging Computational Pathology AI for Noninvasive Optical Imaging Analysis Without Retraining
Nov 19, 2024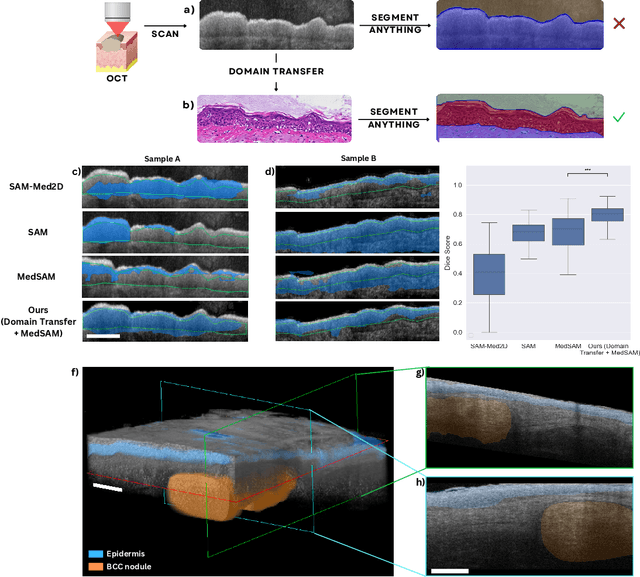
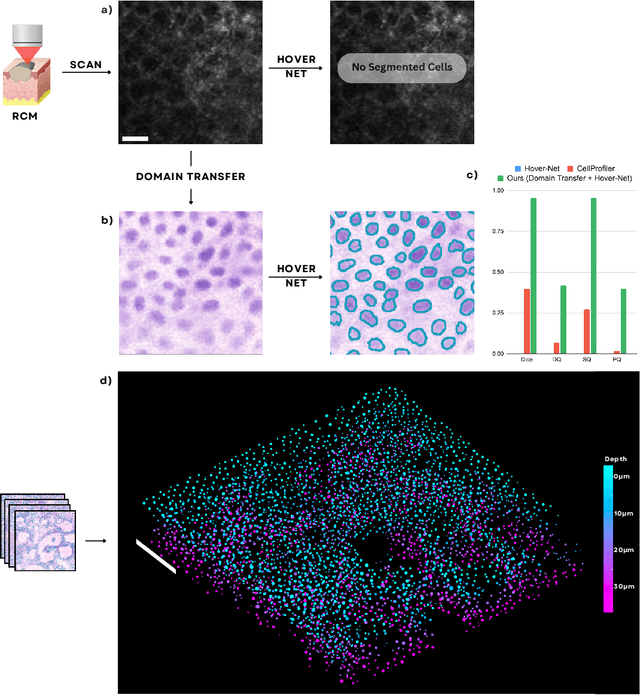
Abstract:Noninvasive optical imaging modalities can probe patient's tissue in 3D and over time generate gigabytes of clinically relevant data per sample. There is a need for AI models to analyze this data and assist clinical workflow. The lack of expert labelers and the large dataset required (>100,000 images) for model training and tuning are the main hurdles in creating foundation models. In this paper we introduce FoundationShift, a method to apply any AI model from computational pathology without retraining. We show our method is more accurate than state of the art models (SAM, MedSAM, SAM-Med2D, CellProfiler, Hover-Net, PLIP, UNI and ChatGPT), with multiple imaging modalities (OCT and RCM). This is achieved without the need for model retraining or fine-tuning. Applying our method to noninvasive in vivo images could enable physicians to readily incorporate optical imaging modalities into their clinical practice, providing real time tissue analysis and improving patient care.
Optimal Shrinkage of Singular Values Under Random Data Contamination
Nov 18, 2017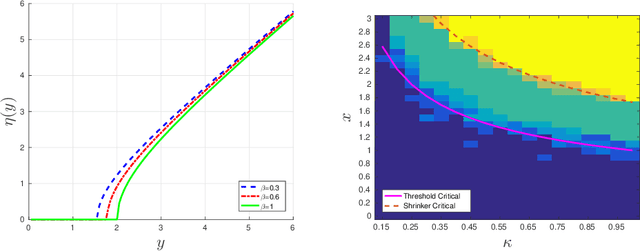
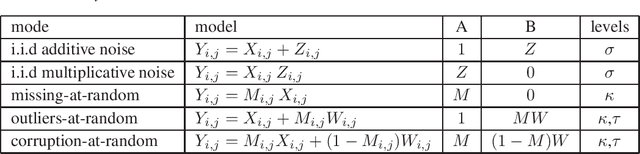
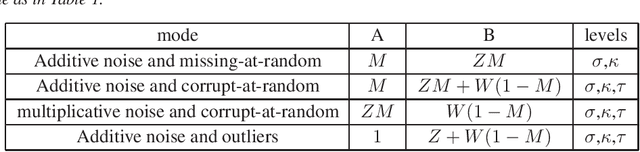
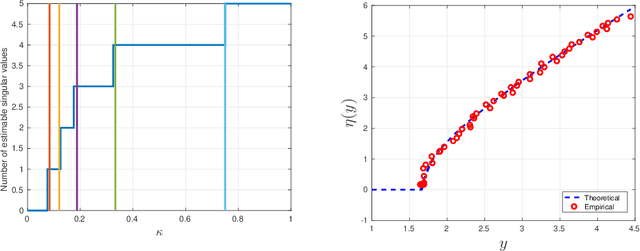
Abstract:A low rank matrix X has been contaminated by uniformly distributed noise, missing values, outliers and corrupt entries. Reconstruction of X from the singular values and singular vectors of the contaminated matrix Y is a key problem in machine learning, computer vision and data science. In this paper we show that common contamination models (including arbitrary combinations of uniform noise,missing values, outliers and corrupt entries) can be described efficiently using a single framework. We develop an asymptotically optimal algorithm that estimates X by manipulation of the singular values of Y , which applies to any of the contamination models considered. Finally, we find an explicit signal-to-noise cutoff, below which estimation of X from the singular value decomposition of Y must fail, in a well-defined sense.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge