Claus-C. Glüer
Opportunistic hip fracture risk prediction in Men from X-ray: Findings from the Osteoporosis in Men (MrOS) Study
Jul 22, 2022



Abstract:Osteoporosis is a common disease that increases fracture risk. Hip fractures, especially in elderly people, lead to increased morbidity, decreased quality of life and increased mortality. Being a silent disease before fracture, osteoporosis often remains undiagnosed and untreated. Areal bone mineral density (aBMD) assessed by dual-energy X-ray absorptiometry (DXA) is the gold-standard method for osteoporosis diagnosis and hence also for future fracture prediction (prognostic). However, the required special equipment is not broadly available everywhere, in particular not to patients in developing countries. We propose a deep learning classification model (FORM) that can directly predict hip fracture risk from either plain radiographs (X-ray) or 2D projection images of computed tomography (CT) data. Our method is fully automated and therefore well suited for opportunistic screening settings, identifying high risk patients in a broader population without additional screening. FORM was trained and evaluated on X-rays and CT projections from the Osteoporosis in Men (MrOS) study. 3108 X-rays (89 incident hip fractures) or 2150 CTs (80 incident hip fractures) with a 80/20 split were used. We show that FORM can correctly predict the 10-year hip fracture risk with a validation AUC of 81.44 +- 3.11% / 81.04 +- 5.54% (mean +- STD) including additional information like age, BMI, fall history and health background across a 5-fold cross validation on the X-ray and CT cohort, respectively. Our approach significantly (p < 0.01) outperforms previous methods like Cox Proportional-Hazards Model and \frax with 70.19 +- 6.58 and 74.72 +- 7.21 respectively on the X-ray cohort. Our model outperform on both cohorts hip aBMD based predictions. We are confident that FORM can contribute on improving osteoporosis diagnosis at an early stage.
An Analysis by Synthesis Method that Allows Accurate Spatial Modeling of Thickness of Cortical Bone from Clinical QCT
Sep 18, 2020



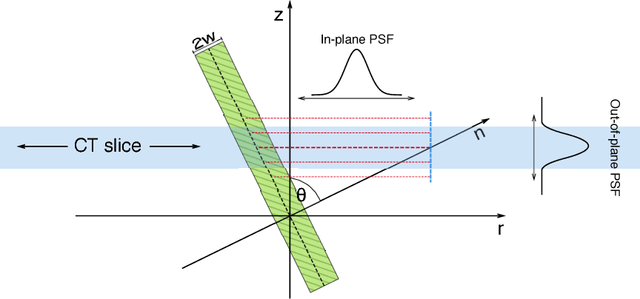
Abstract:Osteoporosis is a skeletal disorder that leads to increased fracture risk due to decreased strength of cortical and trabecular bone. Even with state-of-the-art non-invasive assessment methods there is still a high underdiagnosis rate. Quantitative computed tomography (QCT) permits the selective analysis of cortical bone, however the low spatial resolution of clinical QCT leads to an overestimation of the thickness of cortical bone (Ct.Th) and bone strength. We propose a novel, model based, fully automatic image analysis method that allows accurate spatial modeling of the thickness distribution of cortical bone from clinical QCT. In an analysis-by-synthesis (AbS) fashion a stochastic scan is synthesized from a probabilistic bone model, the optimal model parameters are estimated using a maximum a-posteriori approach. By exploiting the different characteristics of in-plane and out-of-plane point spread functions of CT scanners the proposed method is able assess the spatial distribution of cortical thickness. The method was evaluated on eleven cadaveric human vertebrae, scanned by clinical QCT and analyzed using standard methods and AbS, both compared to high resolution peripheral QCT (HR-pQCT) as gold standard. While standard QCT based measurements overestimated Ct.Th. by 560% and did not show significant correlation with the gold standard ($r^2 = 0.20,\, p = 0.169$) the proposed method eliminated the overestimation and showed a significant tight correlation with the gold standard ($r^2 = 0.98,\, p < 0.0001$) a root mean square error below 10%.
2D and 3D Segmentation of uncertain local collagen fiber orientations in SHG microscopy
Jul 30, 2019



Abstract:Collagen fiber orientations in bones, visible with Second Harmonic Generation (SHG) microscopy, represent the inner structure and its alteration due to influences like cancer. While analyses of these orientations are valuable for medical research, it is not feasible to analyze the needed large amounts of local orientations manually. Since we have uncertain borders for these local orientations only rough regions can be segmented instead of a pixel-wise segmentation. We analyze the effect of these uncertain borders on human performance by a user study. Furthermore, we compare a variety of 2D and 3D methods such as classical approaches like Fourier analysis with state-of-the-art deep neural networks for the classification of local fiber orientations. We present a general way to use pretrained 2D weights in 3D neural networks, such as Inception-ResNet-3D a 3D extension of Inception-ResNet-v2. In a 10 fold cross-validation our two stage segmentation based on Inception-ResNet-3D and transferred 2D ImageNet weights achieves a human comparable accuracy.
An Analysis by Synthesis Approach for Automatic Vertebral Shape Identification in Clinical QCT
Dec 03, 2018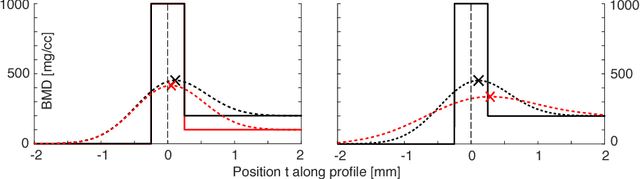
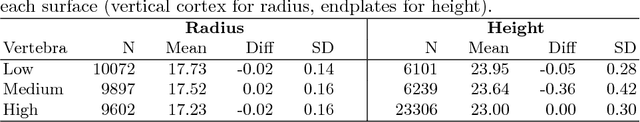
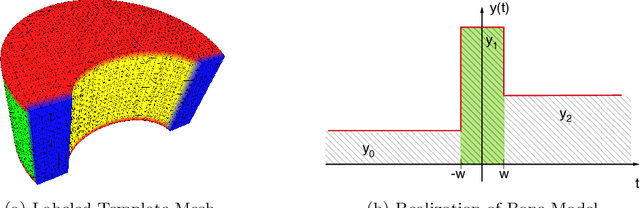
Abstract:Quantitative computed tomography (QCT) is a widely used tool for osteoporosis diagnosis and monitoring. The assessment of cortical markers like cortical bone mineral density (BMD) and thickness is a demanding task, mainly because of the limited spatial resolution of QCT. We propose a direct model based method to automatically identify the surface through the center of the cortex of human vertebra. We develop a statistical bone model and analyze its probability distribution after the imaging process. Using an as-rigid-as-possible deformation we find the cortical surface that maximizes the likelihood of our model given the input volume. Using the European Spine Phantom (ESP) and a high resolution \mu CT scan of a cadaveric vertebra, we show that the proposed method is able to accurately identify the real center of cortex ex-vivo. To demonstrate the in-vivo applicability of our method we use manually obtained surfaces for comparison.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge