Christina Unterberg-Buchwald
Self Supervised Learning for Improved Calibrationless Radial MRI with NLINV-Net
Feb 09, 2024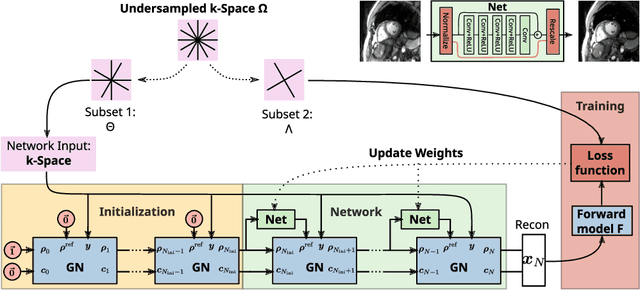
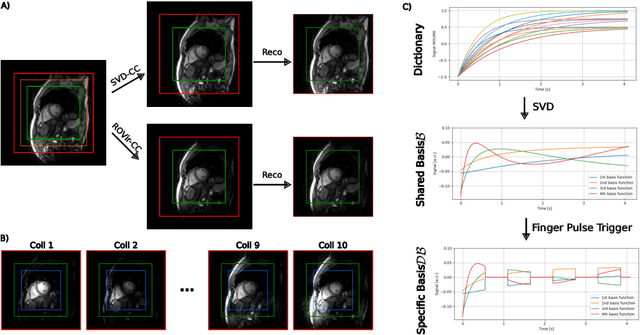
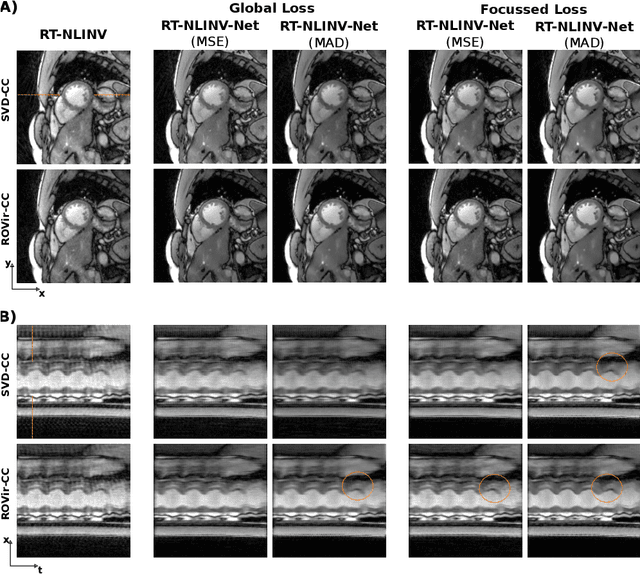
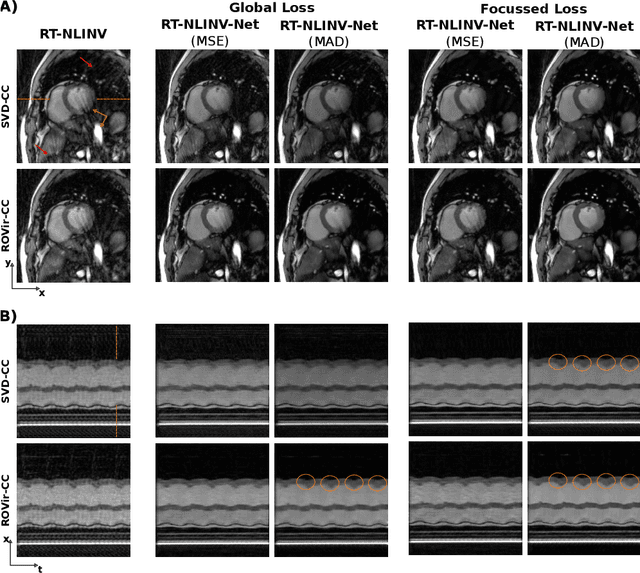
Abstract:Purpose: To develop a neural network architecture for improved calibrationless reconstruction of radial data when no ground truth is available for training. Methods: NLINV-Net is a model-based neural network architecture that directly estimates images and coil sensitivities from (radial) k-space data via non-linear inversion (NLINV). Combined with a training strategy using self-supervision via data undersampling (SSDU), it can be used for imaging problems where no ground truth reconstructions are available. We validated the method for (1) real-time cardiac imaging and (2) single-shot subspace-based quantitative T1 mapping. Furthermore, region-optimized virtual (ROVir) coils were used to suppress artifacts stemming from outside the FoV and to focus the k-space based SSDU loss on the region of interest. NLINV-Net based reconstructions were compared with conventional NLINV and PI-CS (parallel imaging + compressed sensing) reconstruction and the effect of the region-optimized virtual coils and the type of training loss was evaluated qualitatively. Results: NLINV-Net based reconstructions contain significantly less noise than the NLINV-based counterpart. ROVir coils effectively suppress streakings which are not suppressed by the neural networks while the ROVir-based focussed loss leads to visually sharper time series for the movement of the myocardial wall in cardiac real-time imaging. For quantitative imaging, T1-maps reconstructed using NLINV-Net show similar quality as PI-CS reconstructions, but NLINV-Net does not require slice-specific tuning of the regularization parameter. Conclusion: NLINV-Net is a versatile tool for calibrationless imaging which can be used in challenging imaging scenarios where a ground truth is not available.
Assessment of Deep Learning Segmentation for Real-Time Free-Breathing Cardiac Magnetic Resonance Imaging
Dec 01, 2023Abstract:In recent years, a variety of deep learning networks for cardiac MRI (CMR) segmentation have been developed and analyzed. However, nearly all of them are focused on cine CMR under breathold. In this work, accuracy of deep learning methods is assessed for volumetric analysis (via segmentation) of the left ventricle in real-time free-breathing CMR at rest and under exercise stress. Data from healthy volunteers (n=15) for cine and real-time free-breathing CMR were analyzed retrospectively. Segmentations of a commercial software (comDL) and a freely available neural network (nnU-Net), were compared to a reference created via the manual correction of comDL segmentation. Segmentation of left ventricular endocardium (LV), left ventricular myocardium (MYO), and right ventricle (RV) is evaluated for both end-systolic and end-diastolic phases and analyzed with Dice's coefficient (DC). The volumetric analysis includes LV end-diastolic volume (EDV), LV end-systolic volume (ESV), and LV ejection fraction (EF). For cine CMR, nnU-Net and comDL achieve a DC above 0.95 for LV and 0.9 for MYO, and RV. For real-time CMR, the accuracy of nnU-Net exceeds that of comDL overall. For real-time CMR at rest, nnU-Net achieves a DC of 0.94 for LV, 0.89 for MYO, and 0.90 for RV; mean absolute differences between nnU-Net and reference are 2.9mL for EDV, 3.5mL for ESV and 2.6% for EF. For real-time CMR under exercise stress, nnU-Net achieves a DC of 0.92 for LV, 0.85 for MYO, and 0.83 for RV; mean absolute differences between nnU-Net and reference are 11.4mL for EDV, 2.9mL for ESV and 3.6% for EF. Deep learning methods designed or trained for cine CMR segmentation can perform well on real-time CMR. For real-time free-breathing CMR at rest, the performance of deep learning methods is comparable to inter-observer variability in cine CMR and is usable or fully automatic segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge