Christian Sorg
MLV$^2$-Net: Rater-Based Majority-Label Voting for Consistent Meningeal Lymphatic Vessel Segmentation
Nov 13, 2024



Abstract:Meningeal lymphatic vessels (MLVs) are responsible for the drainage of waste products from the human brain. An impairment in their functionality has been associated with aging as well as brain disorders like multiple sclerosis and Alzheimer's disease. However, MLVs have only recently been described for the first time in magnetic resonance imaging (MRI), and their ramified structure renders manual segmentation particularly difficult. Further, as there is no consistent notion of their appearance, human-annotated MLV structures contain a high inter-rater variability that most automatic segmentation methods cannot take into account. In this work, we propose a new rater-aware training scheme for the popular nnU-Net model, and we explore rater-based ensembling strategies for accurate and consistent segmentation of MLVs. This enables us to boost nnU-Net's performance while obtaining explicit predictions in different annotation styles and a rater-based uncertainty estimation. Our final model, MLV$^2$-Net, achieves a Dice similarity coefficient of 0.806 with respect to the human reference standard. The model further matches the human inter-rater reliability and replicates age-related associations with MLV volume.
Complex Grey Matter Structure Segmentation in Brains via Deep Learning: Example of the Claustrum
Aug 08, 2020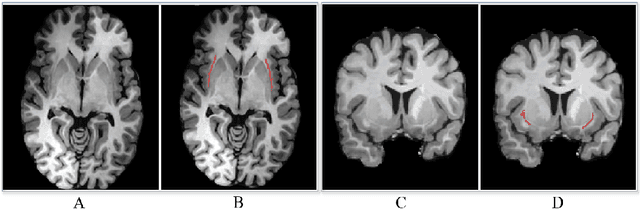
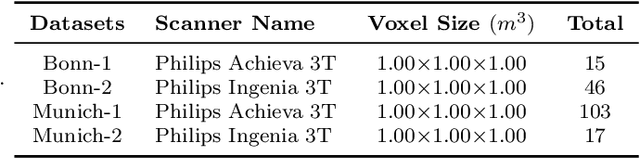
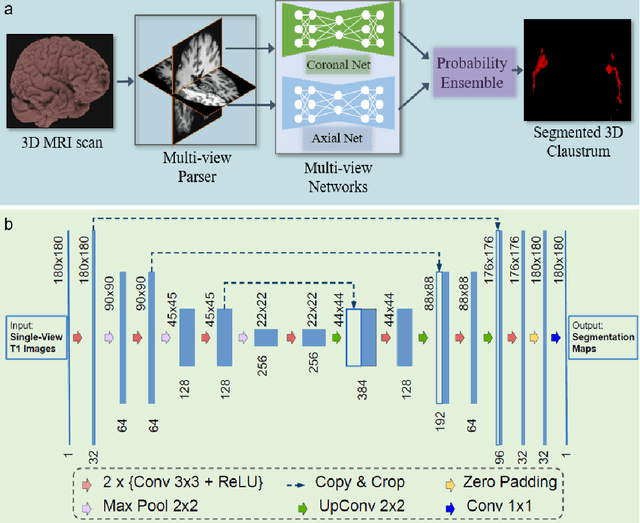

Abstract:Segmentationand parcellation of the brain has been widely performed on brain MRI using atlas-based methods. However, segmentation of the claustrum, a thin and sheet-like structure between insular cortex and putamen has not been amenable to automatized segmentation, thus limiting its investigation in larger imaging cohorts. Recently, deep-learning based approaches have been introduced for automated segmentation of brain structures, yielding great potential to overcome preexisting limitations. In the following, we present a multi-view deep-learning based approach to segment the claustrum in T1-weighted MRI scans. We trained and evaluated the proposed method on 181 manual bilateral claustrum annotations by an expert neuroradiologist serving as reference standard. Cross-validation experiments yielded median volumetric similarity, robust Hausdor? distance and Dice score of 93.3%, 1.41mm and 71.8% respectively which represents equal or superior segmentation performance compared to human intra-rater reliability. Leave-one-scanner-out evaluation showed good transfer-ability of the algorithm to images from unseen scanners, however at slightly inferior performance. Furthermore, we found that AI-based claustrum segmentation benefits from multi-view information and requires sample sizes of around 75 MRI scans in the training set. In conclusion, the developed algorithm has large potential in independent study cohorts and to facilitate MRI-based research of the human claustrum through automated segmentation. The software and models of our method are made publicly available.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge