Christian F. Beckmann
To which reference class do you belong? Measuring racial fairness of reference classes with normative modeling
Jul 26, 2024



Abstract:Reference classes in healthcare establish healthy norms, such as pediatric growth charts of height and weight, and are used to chart deviations from these norms which represent potential clinical risk. How the demographics of the reference class influence clinical interpretation of deviations is unknown. Using normative modeling, a method for building reference classes, we evaluate the fairness (racial bias) in reference models of structural brain images that are widely used in psychiatry and neurology. We test whether including race in the model creates fairer models. We predict self-reported race using the deviation scores from three different reference class normative models, to better understand bias in an integrated, multivariate sense. Across all of these tasks, we uncover racial disparities that are not easily addressed with existing data or commonly used modeling techniques. Our work suggests that deviations from the norm could be due to demographic mismatch with the reference class, and assigning clinical meaning to these deviations should be done with caution. Our approach also suggests that acquiring more representative samples is an urgent research priority.
Learning Cortical Anomaly through Masked Encoding for Unsupervised Heterogeneity Mapping
Dec 11, 2023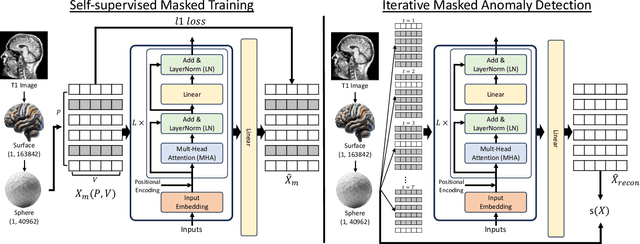
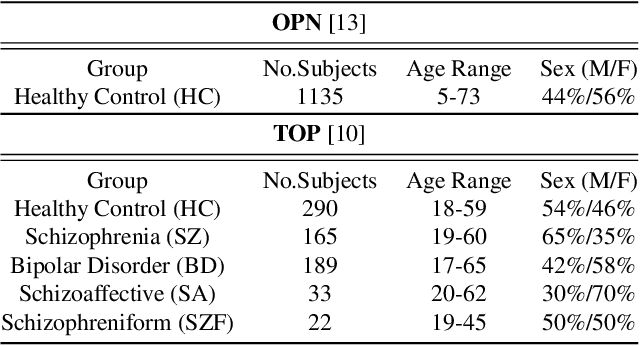

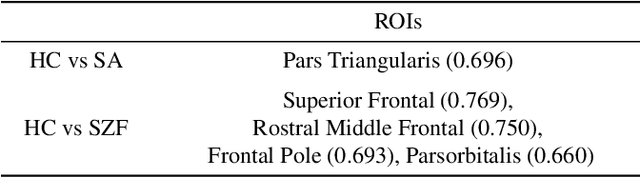
Abstract:The detection of heterogeneous mental disorders based on brain readouts remains challenging due to the complexity of symptoms and the absence of reliable biomarkers. This paper introduces CAM (Cortical Anomaly Detection through Masked Image Modeling), a novel self-supervised framework designed for the unsupervised detection of complex brain disorders using cortical surface features. We employ this framework for the detection of individuals on the psychotic spectrum and demonstrate its capabilities compared to state-ofthe-art methods, achieving an AUC of 0.696 for Schizoaffective and 0.769 for Schizophreniform, without the need for any labels. Furthermore, the analysis of atypical cortical regions includes Pars Triangularis and several frontal areas, often implicated in schizophrenia, provide further confidence in our approach. Altogether, we demonstrate a scalable approach for anomaly detection of complex brain disorders based on cortical abnormalities.
Hierarchical Bayesian Regression for Multi-Site Normative Modeling of Neuroimaging Data
May 25, 2020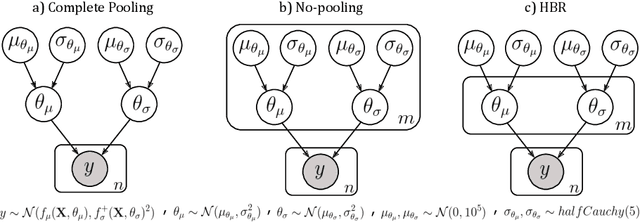
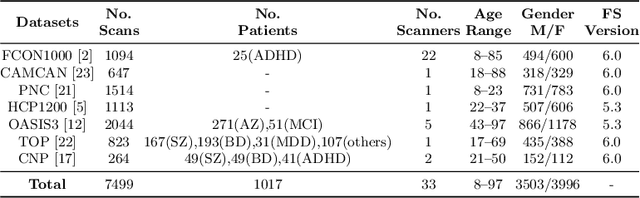
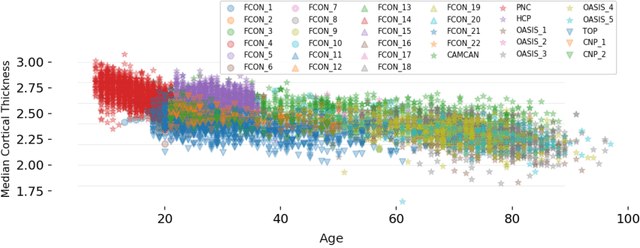
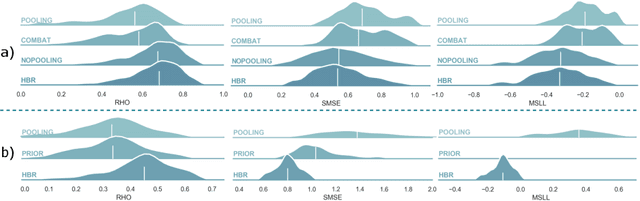
Abstract:Clinical neuroimaging has recently witnessed explosive growth in data availability which brings studying heterogeneity in clinical cohorts to the spotlight. Normative modeling is an emerging statistical tool for achieving this objective. However, its application remains technically challenging due to difficulties in properly dealing with nuisance variation, for example due to variability in image acquisition devices. Here, in a fully probabilistic framework, we propose an application of hierarchical Bayesian regression (HBR) for multi-site normative modeling. Our experimental results confirm the superiority of HBR in deriving more accurate normative ranges on large multi-site neuroimaging data compared to widely used methods. This provides the possibility i) to learn the normative range of structural and functional brain measures on large multi-site data; ii) to recalibrate and reuse the learned model on local small data; therefore, HBR closes the technical loop for applying normative modeling as a medical tool for the diagnosis and prognosis of mental disorders.
Scalable Multi-Task Gaussian Process Tensor Regression for Normative Modeling of Structured Variation in Neuroimaging Data
Jul 31, 2018



Abstract:Most brain disorders are very heterogeneous in terms of their underlying biology and developing analysis methods to model such heterogeneity is a major challenge. A promising approach is to use probabilistic regression methods to estimate normative models of brain function using (f)MRI data then use these to map variation across individuals in clinical populations (e.g., via anomaly detection). To fully capture individual differences, it is crucial to statistically model the patterns of correlation across different brain regions and individuals. However, this is very challenging for neuroimaging data because of high-dimensionality and highly structured patterns of correlation across multiple axes. Here, we propose a general and flexible multi-task learning framework to address this problem. Our model uses a tensor-variate Gaussian process in a Bayesian mixed-effects model and makes use of Kronecker algebra and a low-rank approximation to scale efficiently to multi-way neuroimaging data at the whole brain level. On a publicly available clinical fMRI dataset, we show that our computationally affordable approach substantially improves detection sensitivity over both a mass-univariate normative model and a classifier that --unlike our approach-- has full access to the clinical labels.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge