Carlos A. Silva
Multi-stage Deep Layer Aggregation for Brain Tumor Segmentation
Jan 02, 2021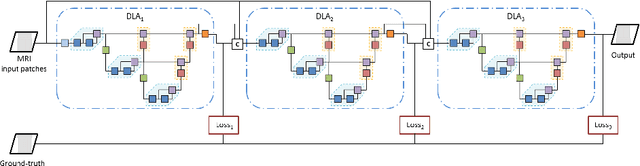
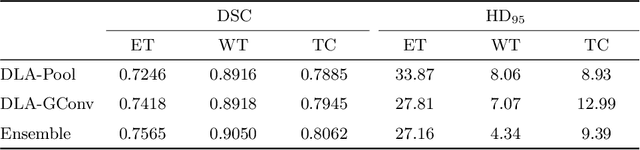
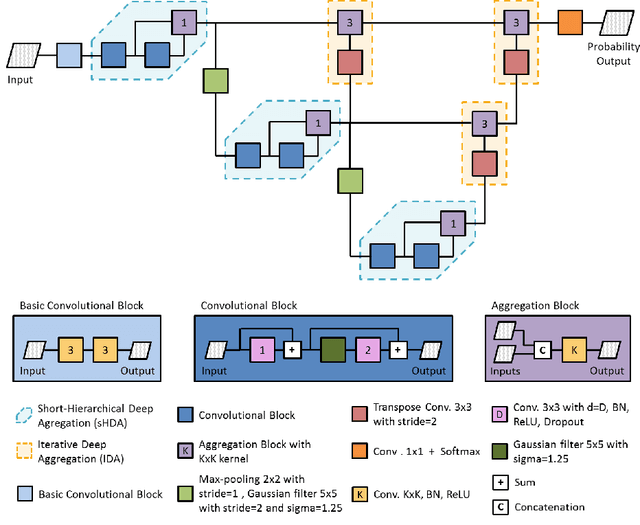
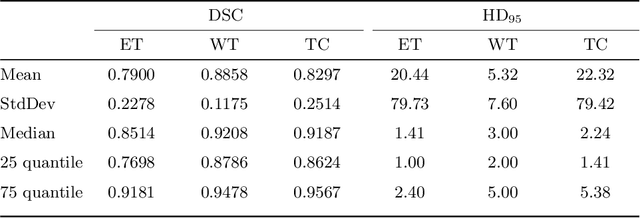
Abstract:Gliomas are among the most aggressive and deadly brain tumors. This paper details the proposed Deep Neural Network architecture for brain tumor segmentation from Magnetic Resonance Images. The architecture consists of a cascade of three Deep Layer Aggregation neural networks, where each stage elaborates the response using the feature maps and the probabilities of the previous stage, and the MRI channels as inputs. The neuroimaging data are part of the publicly available Brain Tumor Segmentation (BraTS) 2020 challenge dataset, where we evaluated our proposal in the BraTS 2020 Validation and Test sets. In the Test set, the experimental results achieved a Dice score of 0.8858, 0.8297 and 0.7900, with an Hausdorff Distance of 5.32 mm, 22.32 mm and 20.44 mm for the whole tumor, core tumor and enhanced tumor, respectively.
Combining unsupervised and supervised learning for predicting the final stroke lesion
Jan 02, 2021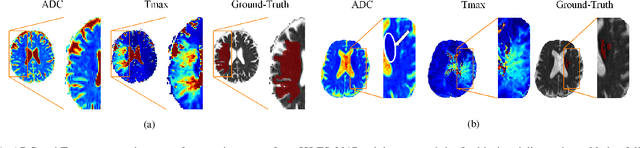
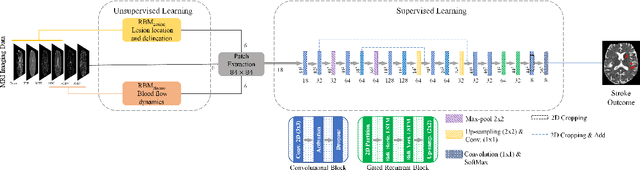
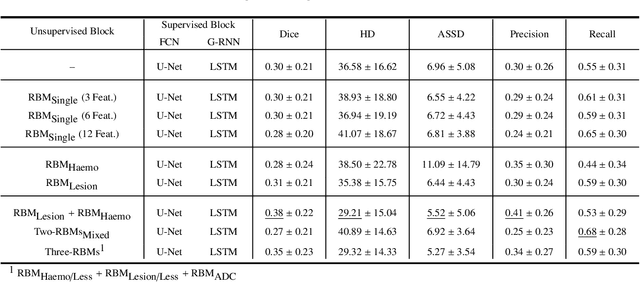
Abstract:Predicting the final ischaemic stroke lesion provides crucial information regarding the volume of salvageable hypoperfused tissue, which helps physicians in the difficult decision-making process of treatment planning and intervention. Treatment selection is influenced by clinical diagnosis, which requires delineating the stroke lesion, as well as characterising cerebral blood flow dynamics using neuroimaging acquisitions. Nonetheless, predicting the final stroke lesion is an intricate task, due to the variability in lesion size, shape, location and the underlying cerebral haemodynamic processes that occur after the ischaemic stroke takes place. Moreover, since elapsed time between stroke and treatment is related to the loss of brain tissue, assessing and predicting the final stroke lesion needs to be performed in a short period of time, which makes the task even more complex. Therefore, there is a need for automatic methods that predict the final stroke lesion and support physicians in the treatment decision process. We propose a fully automatic deep learning method based on unsupervised and supervised learning to predict the final stroke lesion after 90 days. Our aim is to predict the final stroke lesion location and extent, taking into account the underlying cerebral blood flow dynamics that can influence the prediction. To achieve this, we propose a two-branch Restricted Boltzmann Machine, which provides specialized data-driven features from different sets of standard parametric Magnetic Resonance Imaging maps. These data-driven feature maps are then combined with the parametric Magnetic Resonance Imaging maps, and fed to a Convolutional and Recurrent Neural Network architecture. We evaluated our proposal on the publicly available ISLES 2017 testing dataset, reaching a Dice score of 0.38, Hausdorff Distance of 29.21 mm, and Average Symmetric Surface Distance of 5.52 mm.
Adaptive feature recombination and recalibration for semantic segmentation with Fully Convolutional Networks
Jun 19, 2020



Abstract:Fully Convolutional Networks have been achieving remarkable results in image semantic segmentation, while being efficient. Such efficiency results from the capability of segmenting several voxels in a single forward pass. So, there is a direct spatial correspondence between a unit in a feature map and the voxel in the same location. In a convolutional layer, the kernel spans over all channels and extracts information from them. We observe that linear recombination of feature maps by increasing the number of channels followed by compression may enhance their discriminative power. Moreover, not all feature maps have the same relevance for the classes being predicted. In order to learn the inter-channel relationships and recalibrate the channels to suppress the less relevant ones, Squeeze and Excitation blocks were proposed in the context of image classification with Convolutional Neural Networks. However, this is not well adapted for segmentation with Fully Convolutional Networks since they segment several objects simultaneously, hence a feature map may contain relevant information only in some locations. In this paper, we propose recombination of features and a spatially adaptive recalibration block that is adapted for semantic segmentation with Fully Convolutional Networks - the SegSE block. Feature maps are recalibrated by considering the cross-channel information together with spatial relevance. Experimental results indicate that Recombination and Recalibration improve the results of a competitive baseline, and generalize across three different problems: brain tumor segmentation, stroke penumbra estimation, and ischemic stroke lesion outcome prediction. The obtained results are competitive or outperform the state of the art in the three applications.
* Published in IEEE Transactions on Medical Imaging (TMI)
Retinal vessel segmentation based on Fully Convolutional Neural Networks
Dec 19, 2018



Abstract:The retinal vascular condition is a reliable biomarker of several ophthalmologic and cardiovascular diseases, so automatic vessel segmentation may be crucial to diagnose and monitor them. In this paper, we propose a novel method that combines the multiscale analysis provided by the Stationary Wavelet Transform with a multiscale Fully Convolutional Neural Network to cope with the varying width and direction of the vessel structure in the retina. Our proposal uses rotation operations as the basis of a joint strategy for both data augmentation and prediction, which allows us to explore the information learned during training to refine the segmentation. The method was evaluated on three publicly available databases, achieving an average accuracy of 0.9576, 0.9694, and 0.9653, and average area under the ROC curve of 0.9821, 0.9905, and 0.9855 on the DRIVE, STARE, and CHASE_DB1 databases, respectively. It also appears to be robust to the training set and to the inter-rater variability, which shows its potential for real-world applications.
* Support repository for this work: https://github.com/americofmoliveira/VesselSegmentation_ESWA
Automatic brain tumor grading from MRI data using convolutional neural networks and quality assessment
Sep 25, 2018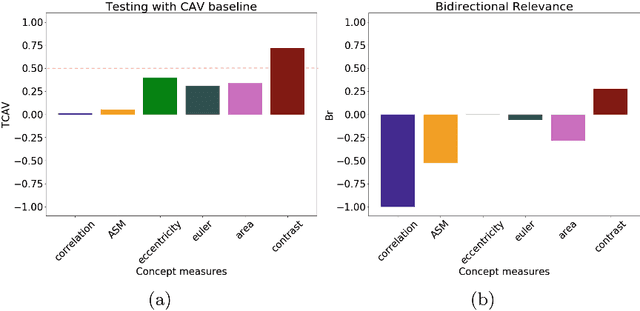
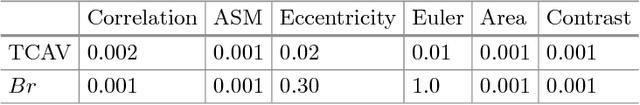
Abstract:Glioblastoma Multiforme is a high grade, very aggressive, brain tumor, with patients having a poor prognosis. Lower grade gliomas are less aggressive, but they can evolve into higher grade tumors over time. Patient management and treatment can vary considerably with tumor grade, ranging from tumor resection followed by a combined radio- and chemotherapy to a "wait and see" approach. Hence, tumor grading is important for adequate treatment planning and monitoring. The gold standard for tumor grading relies on histopathological diagnosis of biopsy specimens. However, this procedure is invasive, time consuming, and prone to sampling error. Given these disadvantages, automatic tumor grading from widely used MRI protocols would be clinically important, as a way to expedite treatment planning and assessment of tumor evolution. In this paper, we propose to use Convolutional Neural Networks for predicting tumor grade directly from imaging data. In this way, we overcome the need for expert annotations of regions of interest. We evaluate two prediction approaches: from the whole brain, and from an automatically defined tumor region. Finally, we employ interpretability methodologies as a quality assurance stage to check if the method is using image regions indicative of tumor grade for classification.
Enhancing clinical MRI Perfusion maps with data-driven maps of complementary nature for lesion outcome prediction
Jun 12, 2018



Abstract:Stroke is the second most common cause of death in developed countries, where rapid clinical intervention can have a major impact on a patient's life. To perform the revascularization procedure, the decision making of physicians considers its risks and benefits based on multi-modal MRI and clinical experience. Therefore, automatic prediction of the ischemic stroke lesion outcome has the potential to assist the physician towards a better stroke assessment and information about tissue outcome. Typically, automatic methods consider the information of the standard kinetic models of diffusion and perfusion MRI (e.g. Tmax, TTP, MTT, rCBF, rCBV) to perform lesion outcome prediction. In this work, we propose a deep learning method to fuse this information with an automated data selection of the raw 4D PWI image information, followed by a data-driven deep-learning modeling of the underlying blood flow hemodynamics. We demonstrate the ability of the proposed approach to improve prediction of tissue at risk before therapy, as compared to only using the standard clinical perfusion maps, hence suggesting on the potential benefits of the proposed data-driven raw perfusion data modelling approach.
Adaptive feature recombination and recalibration for semantic segmentation: application to brain tumor segmentation in MRI
Jun 06, 2018


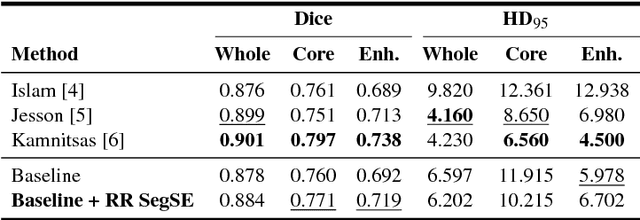
Abstract:Convolutional neural networks (CNNs) have been successfully used for brain tumor segmentation, specifically, fully convolutional networks (FCNs). FCNs can segment a set of voxels at once, having a direct spatial correspondence between units in feature maps (FMs) at a given location and the corresponding classified voxels. In convolutional layers, FMs are merged to create new FMs, so, channel combination is crucial. However, not all FMs have the same relevance for a given class. Recently, in classification problems, Squeeze-and-Excitation (SE) blocks have been proposed to re-calibrate FMs as a whole, and suppress the less informative ones. However, this is not optimal in FCN due to the spatial correspondence between units and voxels. In this article, we propose feature recombination through linear expansion and compression to create more complex features for semantic segmentation. Additionally, we propose a segmentation SE (SegSE) block for feature recalibration that collects contextual information, while maintaining the spatial meaning. Finally, we evaluate the proposed methods in brain tumor segmentation, using publicly available data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge