Can Son Khoo
Multi-Stage Prediction Networks for Data Harmonization
Jul 26, 2019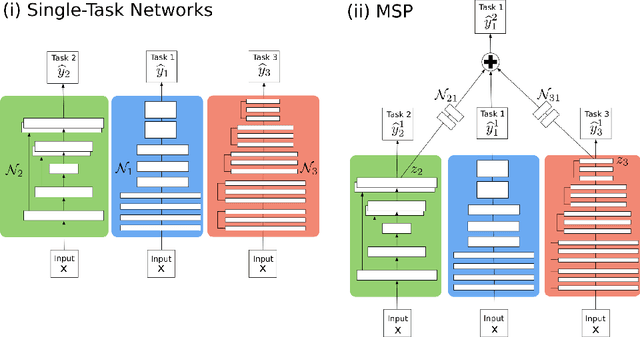
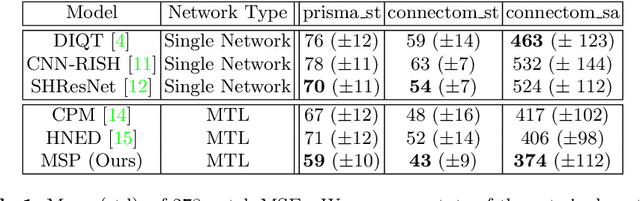
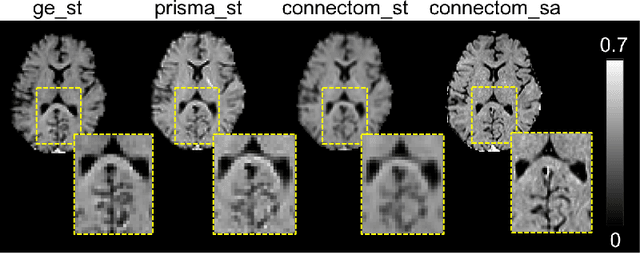
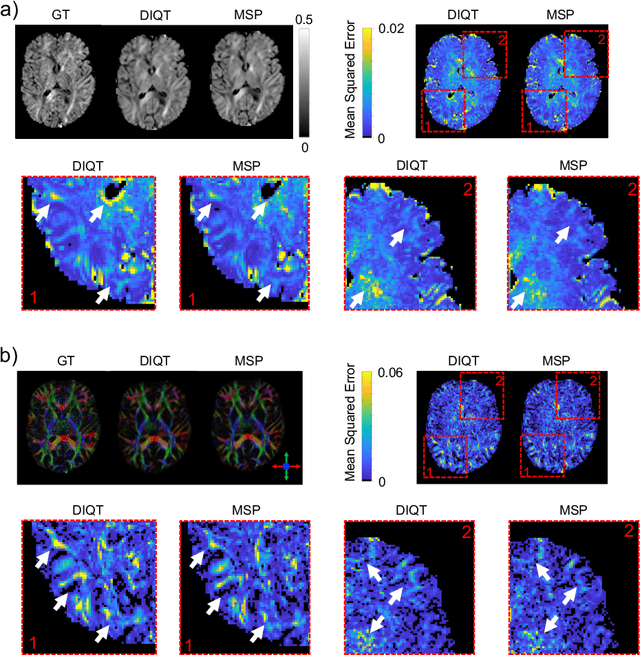
Abstract:In this paper, we introduce multi-task learning (MTL) to data harmonization (DH); where we aim to harmonize images across different acquisition platforms and sites. This allows us to integrate information from multiple acquisitions and improve the predictive performance and learning efficiency of the harmonization model. Specifically, we introduce the Multi Stage Prediction (MSP) Network, a MTL framework that incorporates neural networks of potentially disparate architectures, trained for different individual acquisition platforms, into a larger architecture that is refined in unison. The MSP utilizes high-level features of single networks for individual tasks, as inputs of additional neural networks to inform the final prediction, therefore exploiting redundancy across tasks to make the most of limited training data. We validate our methods on a dMRI harmonization challenge dataset, where we predict three modern platform types, from one obtained from an old scanner. We show how MTL architectures, such as the MSP, produce around 20\% improvement of patch-based mean-squared error over current state-of-the-art methods and that our MSP outperforms off-the-shelf MTL networks. Our code is available https://github.com/sbb-gh/ .
Large-scale mammography CAD with Deformable Conv-Nets
Feb 19, 2019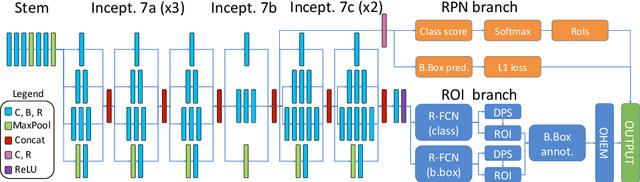
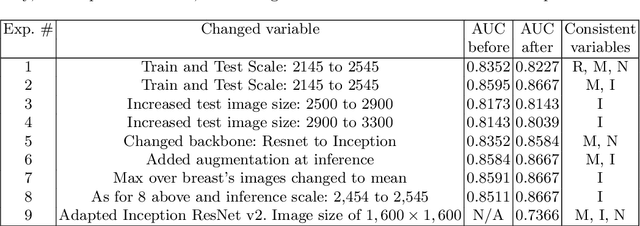
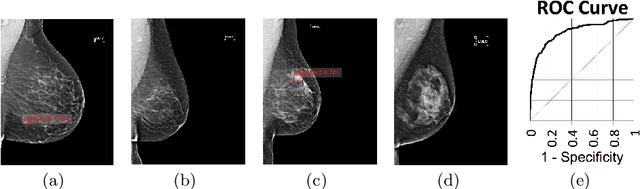
Abstract:State-of-the-art deep learning methods for image processing are evolving into increasingly complex meta-architectures with a growing number of modules. Among them, region-based fully convolutional networks (R-FCN) and deformable convolutional nets (DCN) can improve CAD for mammography: R-FCN optimizes for speed and low consumption of memory, which is crucial for processing the high resolutions of to 50 micrometers used by radiologists. Deformable convolution and pooling can model a wide range of mammographic findings of different morphology and scales, thanks to their versatility. In this study, we present a neural net architecture based on R-FCN / DCN, that we have adapted from the natural image domain to suit mammograms -- particularly their larger image size -- without compromising resolution. We trained the network on a large, recently released dataset (Optimam) including 6,500 cancerous mammograms. By combining our modern architecture with such a rich dataset, we achieved an area under the ROC curve of 0.879 for breast-wise detection in the DREAMS challenge (130,000 withheld images), which surpassed all other submissions in the competitive phase.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge