Cédric Wemmert
HistoStarGAN: A Unified Approach to Stain Normalisation, Stain Transfer and Stain Invariant Segmentation in Renal Histopathology
Oct 18, 2022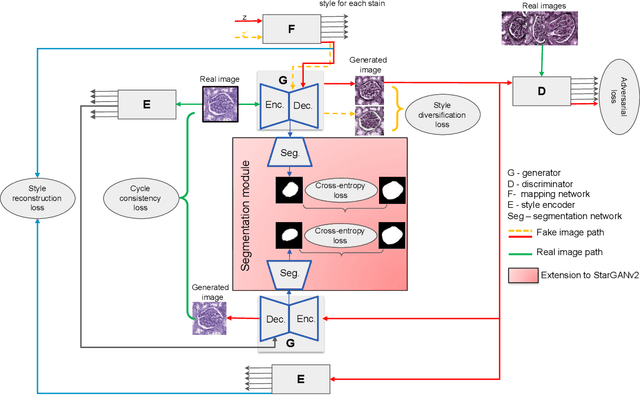

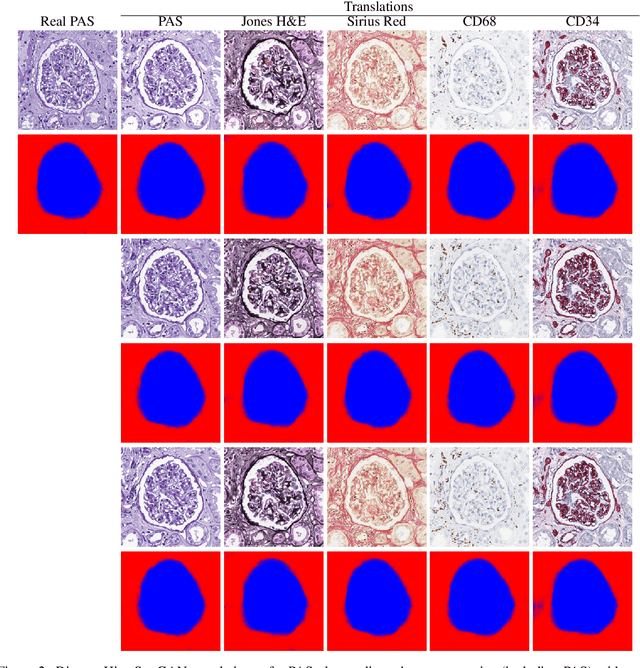
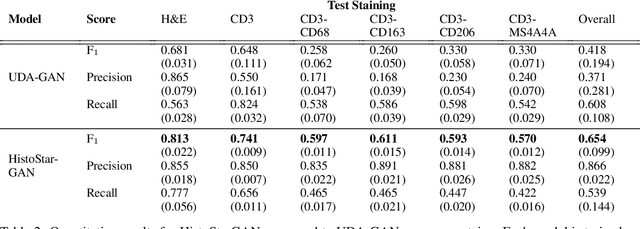
Abstract:Virtual stain transfer is a promising area of research in Computational Pathology, which has a great potential to alleviate important limitations when applying deeplearningbased solutions such as lack of annotations and sensitivity to a domain shift. However, in the literature, the majority of virtual staining approaches are trained for a specific staining or stain combination, and their extension to unseen stainings requires the acquisition of additional data and training. In this paper, we propose HistoStarGAN, a unified framework that performs stain transfer between multiple stainings, stain normalisation and stain invariant segmentation, all in one inference of the model. We demonstrate the generalisation abilities of the proposed solution to perform diverse stain transfer and accurate stain invariant segmentation over numerous unseen stainings, which is the first such demonstration in the field. Moreover, the pre-trained HistoStar-GAN model can serve as a synthetic data generator, which paves the way for the use of fully annotated synthetic image data to improve the training of deep learning-based algorithms. To illustrate the capabilities of our approach, as well as the potential risks in the microscopy domain, inspired by applications in natural images, we generated KidneyArtPathology, a fully annotated artificial image dataset for renal pathology.
Self adversarial attack as an augmentation method for immunohistochemical stainings
Mar 21, 2021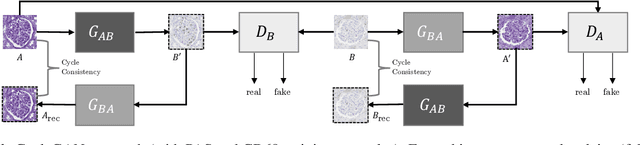
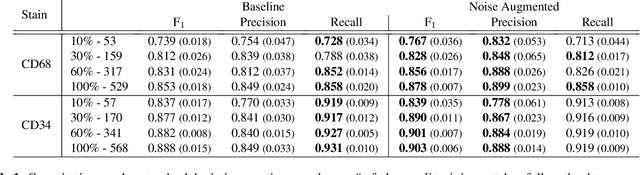

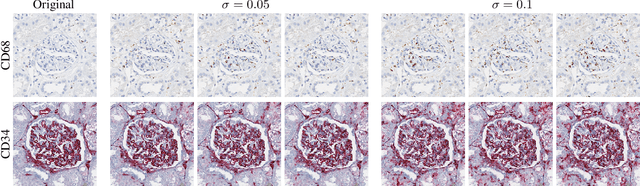
Abstract:It has been shown that unpaired image-to-image translation methods constrained by cycle-consistency hide the information necessary for accurate input reconstruction as imperceptible noise. We demonstrate that, when applied to histopathology data, this hidden noise appears to be related to stain specific features and show that this is the case with two immunohistochemical stainings during translation to Periodic acid- Schiff (PAS), a histochemical staining method commonly applied in renal pathology. Moreover, by perturbing this hidden information, the translation models produce different, plausible outputs. We demonstrate that this property can be used as an augmentation method which, in a case of supervised glomeruli segmentation, leads to improved performance.
Towards Histopathological Stain Invariance by Unsupervised Domain Augmentation using Generative Adversarial Networks
Dec 22, 2020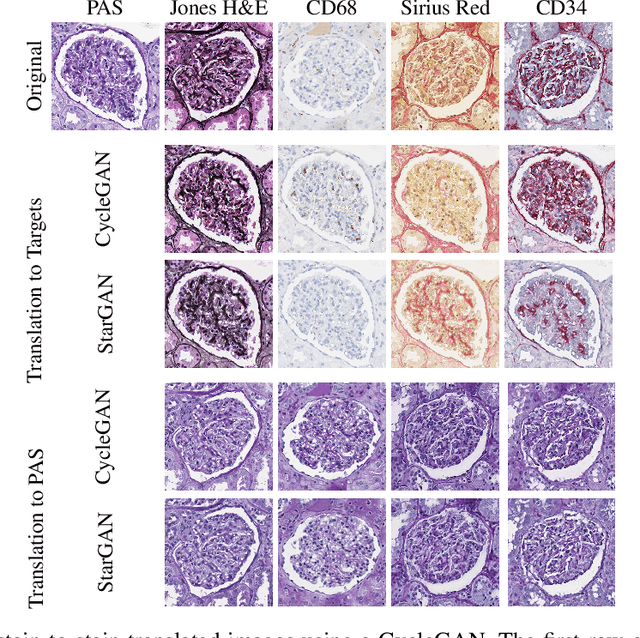
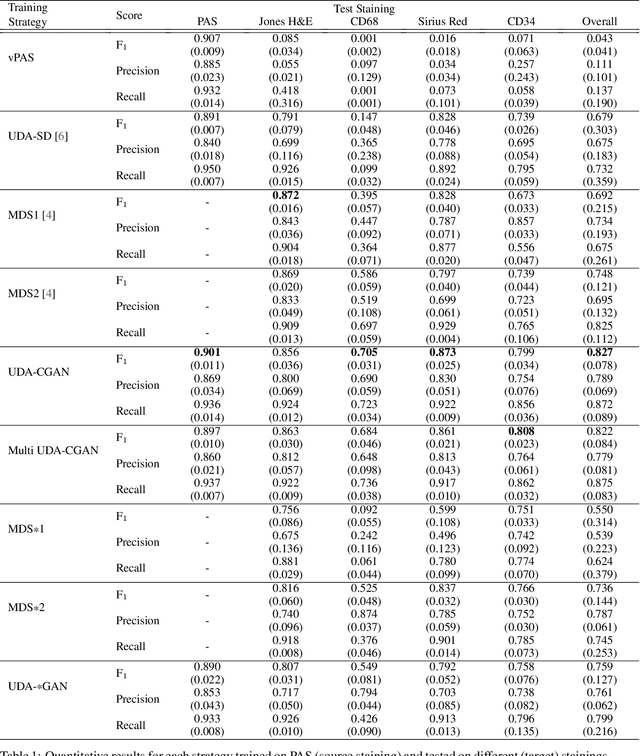
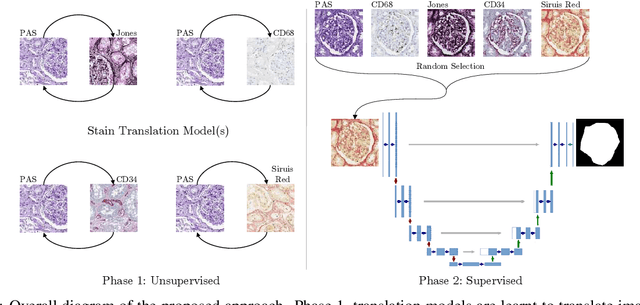
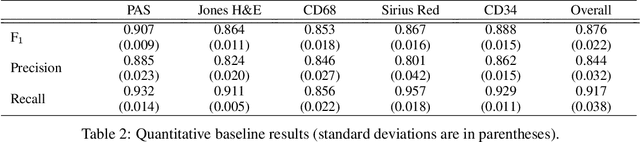
Abstract:The application of supervised deep learning methods in digital pathology is limited due to their sensitivity to domain shift. Digital Pathology is an area prone to high variability due to many sources, including the common practice of evaluating several consecutive tissue sections stained with different staining protocols. Obtaining labels for each stain is very expensive and time consuming as it requires a high level of domain knowledge. In this article, we propose an unsupervised augmentation approach based on adversarial image-to-image translation, which facilitates the training of stain invariant supervised convolutional neural networks. By training the network on one commonly used staining modality and applying it to images that include corresponding, but differently stained, tissue structures, the presented method demonstrates significant improvements over other approaches. These benefits are illustrated in the problem of glomeruli segmentation in seven different staining modalities (PAS, Jones H&E, CD68, Sirius Red, CD34, H&E and CD3) and analysis of the learned representations demonstrate their stain invariance.
An automatic framework to study the tissue micro-environment of renal glomeruli in differently stained consecutive digital whole slide images
Aug 29, 2020



Abstract:Objective: This article presents an automatic image processing framework to extract quantitative high-level information describing the micro-environment of glomeruli in consecutive whole slide images (WSIs) processed with different staining modalities of patients with chronic kidney rejection after kidney transplantation. Methods: This three step framework consists of: 1) cell and anatomical structure segmentation based on colour deconvolution and deep learning 2) fusion of information from different stainings using a newly developed registration algorithm 3) feature extraction. Results: Each step of the framework is validated independently both quantitatively and qualitatively by pathologists. An illustration of the different types of features that can be extracted is presented. Conclusion: The proposed generic framework allows for the analysis of the micro-environment surrounding large structures that can be segmented (either manually or automatically). It is independent of the segmentation approach and is therefore applicable to a variety of biomedical research questions. Significance: Chronic tissue remodelling processes after kidney transplantation can result in interstitial fibrosis and tubular atrophy (IFTA) and glomerulosclerosis. This pipeline provides tools to quantitatively analyse, in the same spatial context, information from different consecutive WSIs and help researchers understand the complex underlying mechanisms leading to IFTA and glomerulosclerosis.
Strategies for Training Stain Invariant CNNs
Nov 03, 2018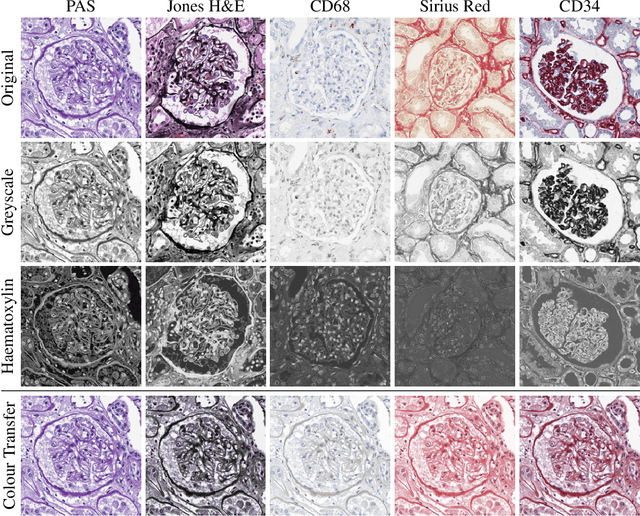
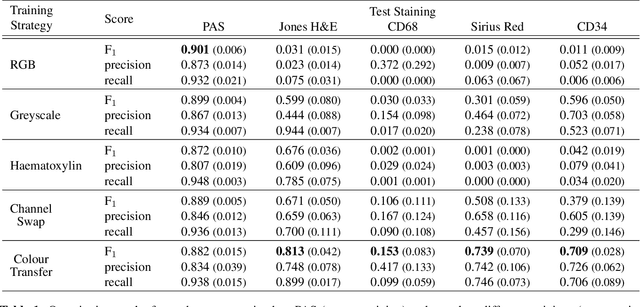
Abstract:An important part of Digital Pathology is the analysis of multiple digitised whole slide images from differently stained tissue sections. It is common practice to mount consecutive sections containing corresponding microscopic structures on glass slides, and to stain them differently to highlight specific tissue components. These multiple staining modalities result in very different images but include a significant amount of consistent image information. Deep learning approaches have recently been proposed to analyse these images in order to automatically identify objects of interest for pathologists. These supervised approaches require a vast amount of annotations, which are difficult and expensive to acquire---a problem that is multiplied with multiple stainings. This article presents several training strategies that make progress towards stain invariant networks. By training the network on one commonly used staining modality and applying it to images that include corresponding but differently stained tissue structures, the presented unsupervised strategies demonstrate significant improvements over standard training strategies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge