Bruno V. Rego
Neural operator learning of heterogeneous mechanobiological insults contributing to aortic aneurysms
May 08, 2022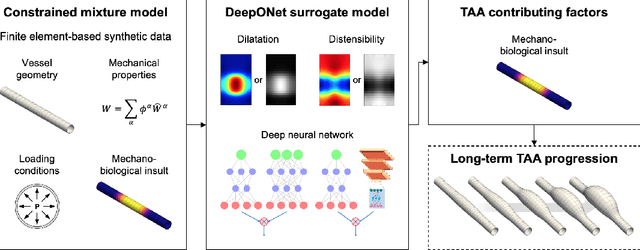
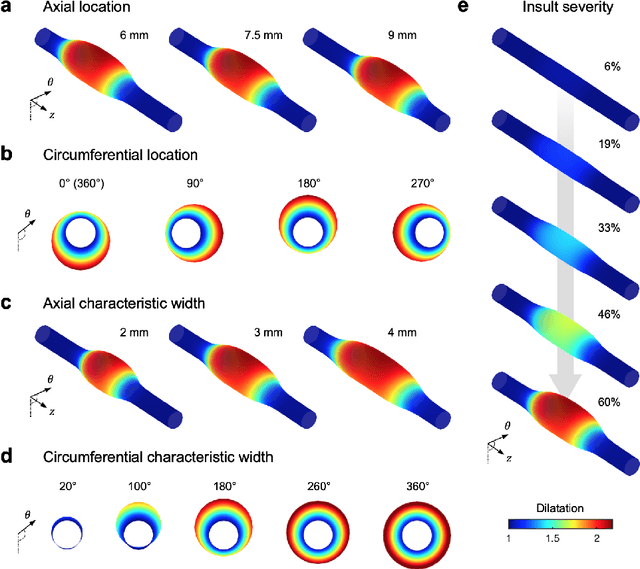

Abstract:Thoracic aortic aneurysm (TAA) is a localized dilatation of the aorta resulting from compromised wall composition, structure, and function, which can lead to life-threatening dissection or rupture. Several genetic mutations and predisposing factors that contribute to TAA have been studied in mouse models to characterize specific changes in aortic microstructure and material properties that result from a wide range of mechanobiological insults. Assessments of TAA progression in vivo is largely limited to measurements of aneurysm size and growth rate. It has been shown that aortic geometry alone is not sufficient to predict the patient-specific progression of TAA but computational modeling of the evolving biomechanics of the aorta could predict future geometry and properties from initiating insults. In this work, we present an integrated framework to train a deep operator network (DeepONet)-based surrogate model to identify contributing factors for TAA by using FE-based datasets of aortic growth and remodeling resulting from prescribed insults. For training data, we investigate multiple types of TAA risk factors and spatial distributions within a constrained mixture model to generate axial--azimuthal maps of aortic dilatation and distensibility. The trained network is then capable of predicting the initial distribution and extent of the insult from a given set of dilatation and distensibility information. Two DeepONet frameworks are proposed, one trained on sparse information and one on full-field grayscale images, to gain insight into a preferred neural operator-based approach. Performance of the surrogate models is evaluated through multiple simulations carried out on insult distributions varying from fusiform to complex. We show that the proposed approach can predict patient-specific mechanobiological insult profile with a high accuracy, particularly when based on full-field images.
Simulating progressive intramural damage leading to aortic dissection using an operator-regression neural network
Aug 25, 2021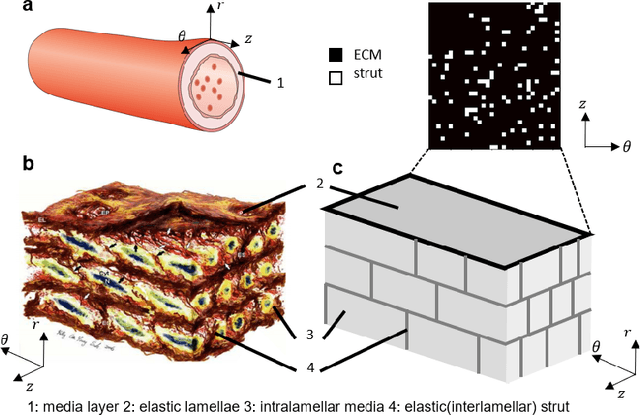
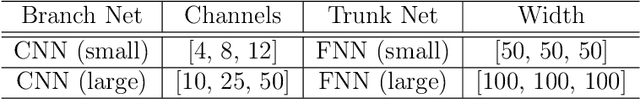


Abstract:Aortic dissection progresses via delamination of the medial layer of the wall. Notwithstanding the complexity of this process, insight has been gleaned by studying in vitro and in silico the progression of dissection driven by quasi-static pressurization of the intramural space by fluid injection, which demonstrates that the differential propensity of dissection can be affected by spatial distributions of structurally significant interlamellar struts that connect adjacent elastic lamellae. In particular, diverse histological microstructures may lead to differential mechanical behavior during dissection, including the pressure--volume relationship of the injected fluid and the displacement field between adjacent lamellae. In this study, we develop a data-driven surrogate model for the delamination process for differential strut distributions using DeepONet, a new operator--regression neural network. The surrogate model is trained to predict the pressure--volume curve of the injected fluid and the damage progression field of the wall given a spatial distribution of struts, with in silico data generated with a phase-field finite element model. The results show that DeepONet can provide accurate predictions for diverse strut distributions, indicating that this composite branch-trunk neural network can effectively extract the underlying functional relationship between distinctive microstructures and their mechanical properties. More broadly, DeepONet can facilitate surrogate model-based analyses to quantify biological variability, improve inverse design, and predict mechanical properties based on multi-modality experimental data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge