Bilwaj Gaonkar
EigenRank by Committee: A Data Subset Selection and Failure Prediction paradigm for Robust Deep Learning based Medical Image Segmentation
Aug 17, 2019
Abstract:Translation of fully automated deep learning based medical image segmentation technologies to clinical workflows face two main algorithmic challenges. The first, is the collection and archival of large quantities of manually annotated ground truth data for both training and validation. The second is the relative inability of the majority of deep learning based segmentation techniques to alert physicians to a likely segmentation failure. Here we propose a novel algorithm, named `Eigenrank' which addresses both of these challenges. Eigenrank can select for manual labeling, a subset of medical images from a large database, such that a U-Net trained on this subset is superior to one trained on a randomly selected subset of the same size. Eigenrank can also be used to pick out, cases in a large database, where deep learning segmentation will fail. We present our algorithm, followed by results and a discussion of how Eigenrank exploits the Von Neumann information to perform both data subset selection and failure prediction for medical image segmentation using deep learning.
Extreme Augmentation : Can deep learning based medical image segmentation be trained using a single manually delineated scan?
Oct 03, 2018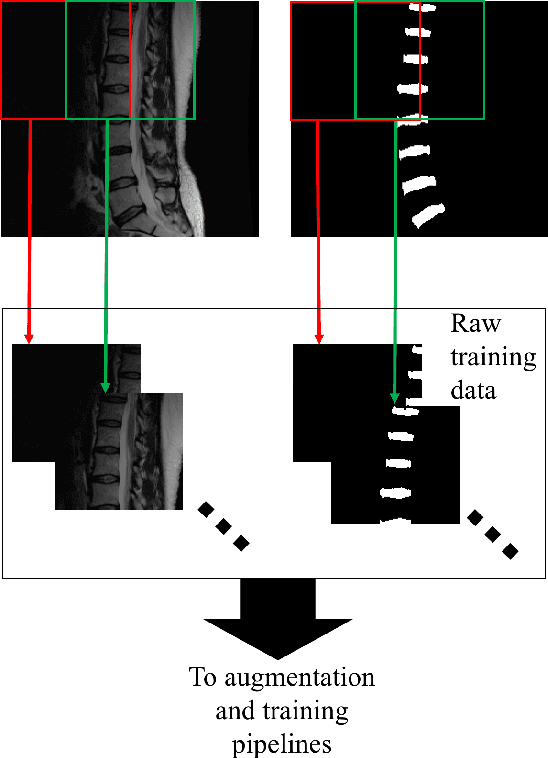
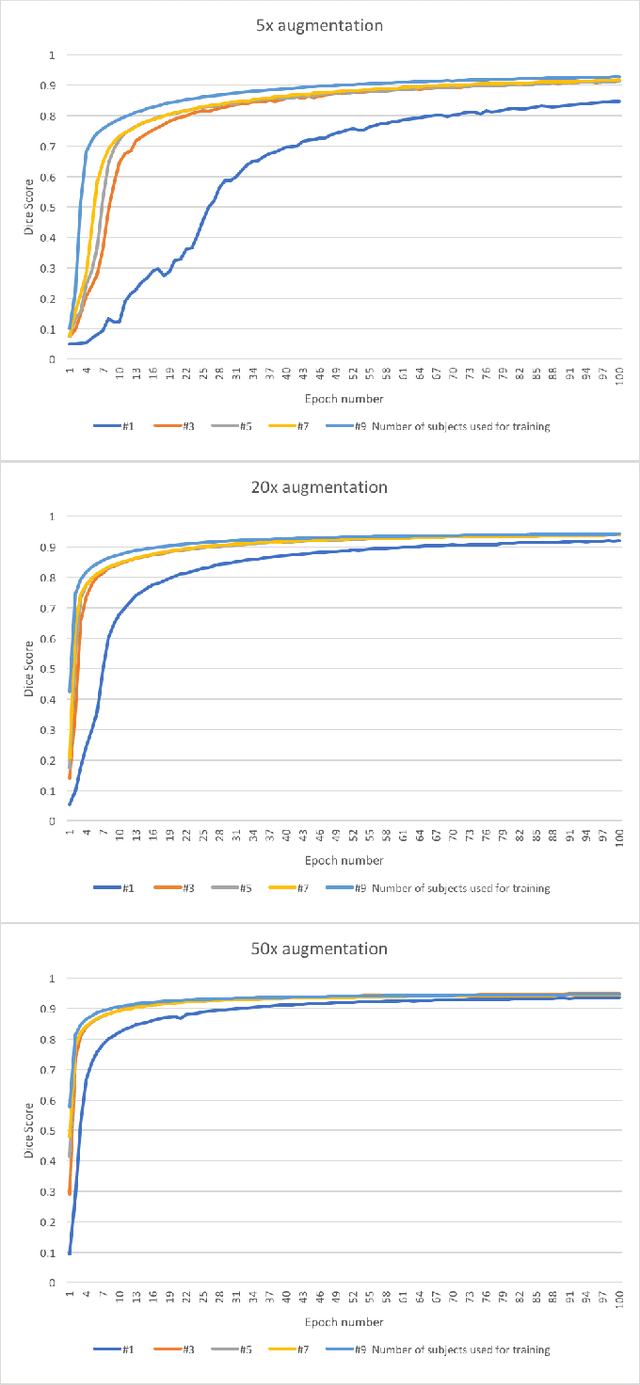
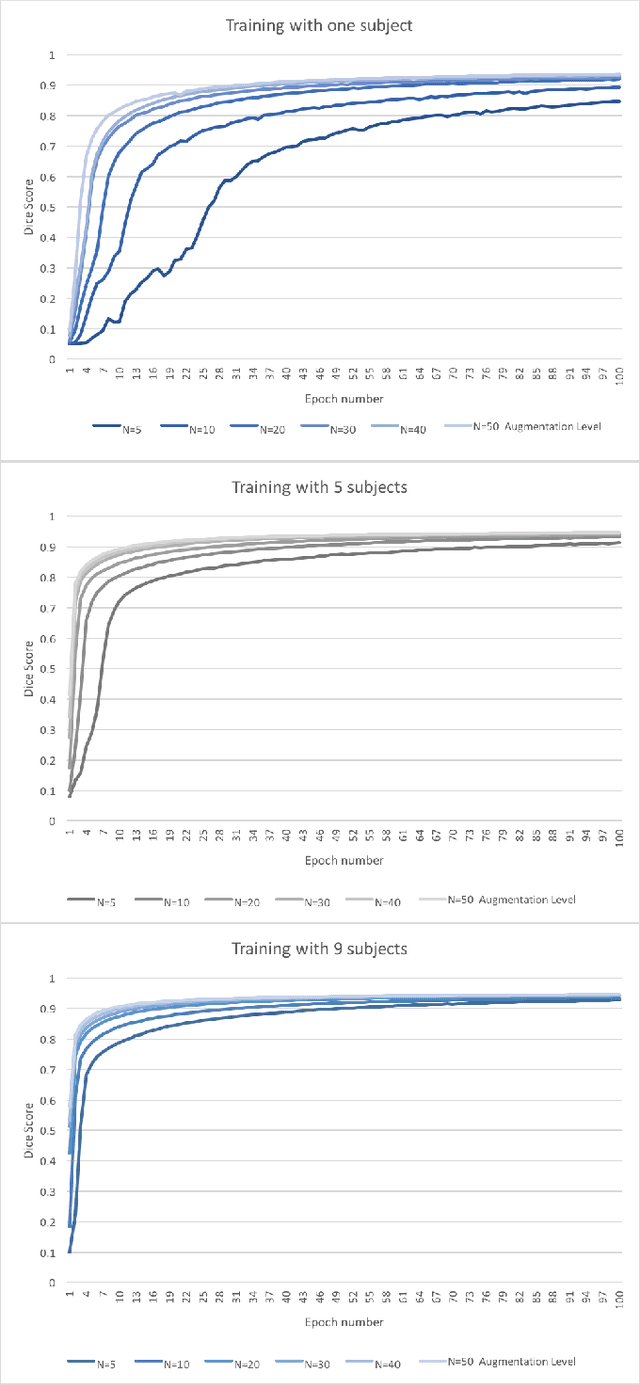
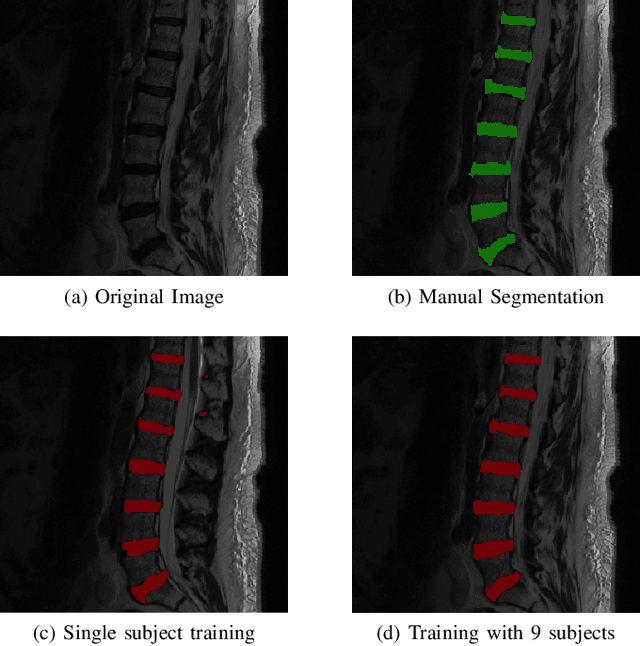
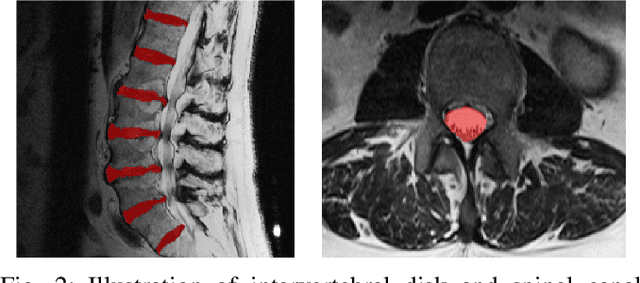
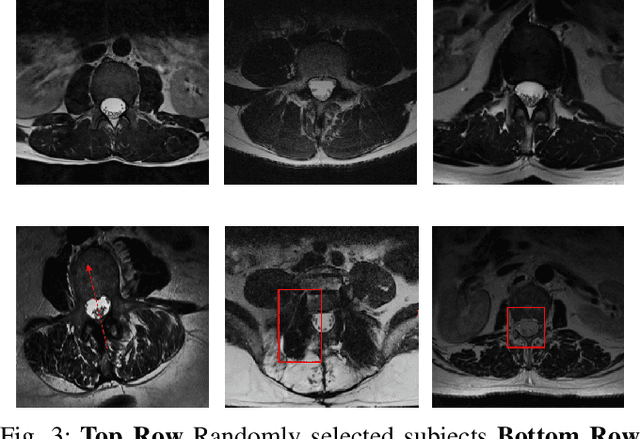
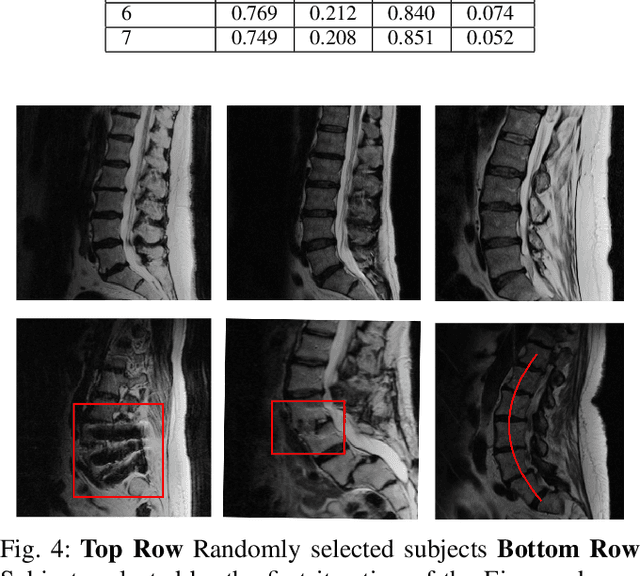
Abstract:Yes, it can. Data augmentation is perhaps the oldest preprocessing step in computer vision literature. Almost every computer vision model trained on imaging data uses some form of augmentation. In this paper, we use the inter-vertebral disk segmentation task alongside a deep residual U-Net as the learning model, to explore the effectiveness of augmentation. In the extreme, we observed that a model trained on patches extracted from just one scan, with each patch augmented 50 times; achieved a Dice score of 0.73 in a validation set of 40 cases. Qualitative evaluation indicated a clinically usable segmentation algorithm, which appropriately segments regions of interest, alongside limited false positive specks. When the initial patches are extracted from nine scans the average Dice coefficient jumps to 0.86 and most of the false positives disappear. While this still falls short of state-of-the-art deep learning based segmentation of discs reported in literature, qualitative examination reveals that it does yield segmentation, which can be amended by expert clinicians with minimal effort to generate additional data for training improved deep models. Extreme augmentation of training data, should thus be construed as a strategy for training deep learning based algorithms, when very little manually annotated data is available to work with. Models trained with extreme augmentation can then be used to accelerate the generation of manually labelled data. Hence, we show that extreme augmentation can be a valuable tool in addressing scaling up small imaging data sets to address medical image segmentation tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge