Benjamin Marty
Upper-body free-breathing Magnetic Resonance Fingerprinting applied to the quantification of water T1 and fat fraction
Sep 24, 2024Abstract:Over the past decade, Magnetic Resonance Fingerprinting (MRF) has emerged as an efficient paradigm for the rapid and simultaneous quantification of multiple MRI parameters, including fat fraction (FF), water T1 ($T1_{H2O}$), water T2 ($T2_{H2O}$), and fat T1 ($T1_{fat}$). These parameters serve as promising imaging biomarkers in various anatomical targets such as the heart, liver, and skeletal muscles. However, measuring these parameters in the upper body poses challenges due to physiological motion, particularly respiratory motion. In this work, we propose a novel approach, motion-corrected (MoCo) MRF T1-FF, which estimates the motion field using an optimized preliminary motion scan and uses it to correct the MRF acquisition data before dictionary search for reconstructing motion-corrected FF and $T1_{H2O}$ parametric maps of the upper-body region. We validated this framework using an $\textit{in vivo}$ dataset comprising ten healthy volunteers and a 10-year-old boy with Duchenne muscular dystrophy. At the ROI level, in regions minimally affected by motion, no significant bias was observed between the uncorrected and MoCo reconstructions for FF (mean difference of -0.7%) and $T1_{H2O}$ (-4.9 ms) values. Moreover, MoCo MRF T1-FF significantly reduced the standard deviations of distributions assessed in these regions, indicating improved precision. Notably, in regions heavily affected by motion, such as respiratory muscles, liver, and kidneys, the MRF parametric maps exhibited a marked reduction in motion blurring and streaking artifacts after motion correction. Furthermore, the diaphragm was consistently discernible on parametric maps after motion correction. This approach lays the groundwork for the joint 3D quantification of FF and $T1_{H2O}$ in regions that are rarely studied, such as the respiratory muscles, particularly the intercostal muscles and diaphragm.
Learning Bloch Simulations for MR Fingerprinting by Invertible Neural Networks
Aug 10, 2020



Abstract:Magnetic resonance fingerprinting (MRF) enables fast and multiparametric MR imaging. Despite fast acquisition, the state-of-the-art reconstruction of MRF based on dictionary matching is slow and lacks scalability. To overcome these limitations, neural network (NN) approaches estimating MR parameters from fingerprints have been proposed recently. Here, we revisit NN-based MRF reconstruction to jointly learn the forward process from MR parameters to fingerprints and the backward process from fingerprints to MR parameters by leveraging invertible neural networks (INNs). As a proof-of-concept, we perform various experiments showing the benefit of learning the forward process, i.e., the Bloch simulations, for improved MR parameter estimation. The benefit especially accentuates when MR parameter estimation is difficult due to MR physical restrictions. Therefore, INNs might be a feasible alternative to the current solely backward-based NNs for MRF reconstruction.
Spatially Regularized Parametric Map Reconstruction for Fast Magnetic Resonance Fingerprinting
Nov 09, 2019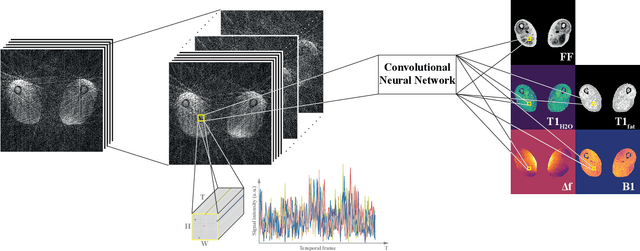

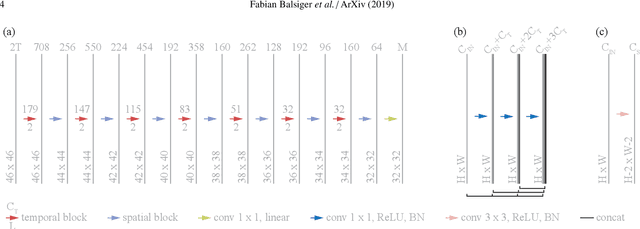

Abstract:Magnetic resonance fingerprinting (MRF) provides a unique concept for simultaneous and fast acquisition of multiple quantitative MR parameters. Despite acquisition efficiency, adoption of MRF into the clinics is hindered by its dictionary-based reconstruction, which is computationally demanding and lacks scalability. Here, we propose a convolutional neural network-based reconstruction, which enables both accurate and fast reconstruction of parametric maps, and is adaptable based on the needs of spatial regularization and the capacity for the reconstruction. We evaluated the method using MRF T1-FF, an MRF sequence for T1 relaxation time of water and fat fraction mapping. We demonstrate the method's performance on a highly heterogeneous dataset consisting of 164 patients with various neuromuscular diseases imaged at thighs and legs. We empirically show the benefit of incorporating spatial regularization during the reconstruction and demonstrate that the method learns meaningful features from MR physics perspective. Further, we investigate the ability of the method to handle highly heterogeneous morphometric variations and its generalization to anatomical regions unseen during training. The obtained results outperform the state-of-the-art in deep learning-based MRF reconstruction. Coupled with fast MRF sequences, the proposed method has the potential of enabling multiparametric MR imaging in clinically feasible time.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge