Benjamin Brinkmann
Integrating Artificial Intelligence with Real-time Intracranial EEG Monitoring to Automate Interictal Identification of Seizure Onset Zones in Focal Epilepsy
Dec 15, 2018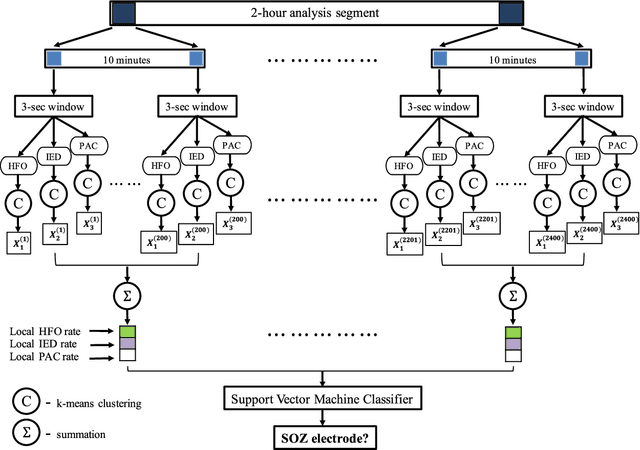

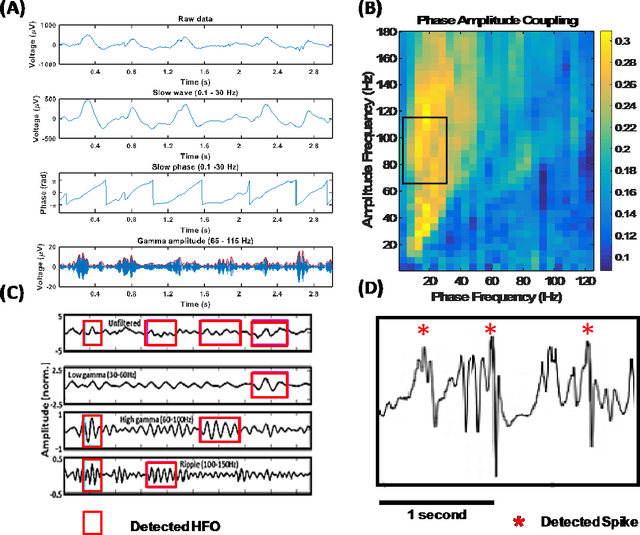
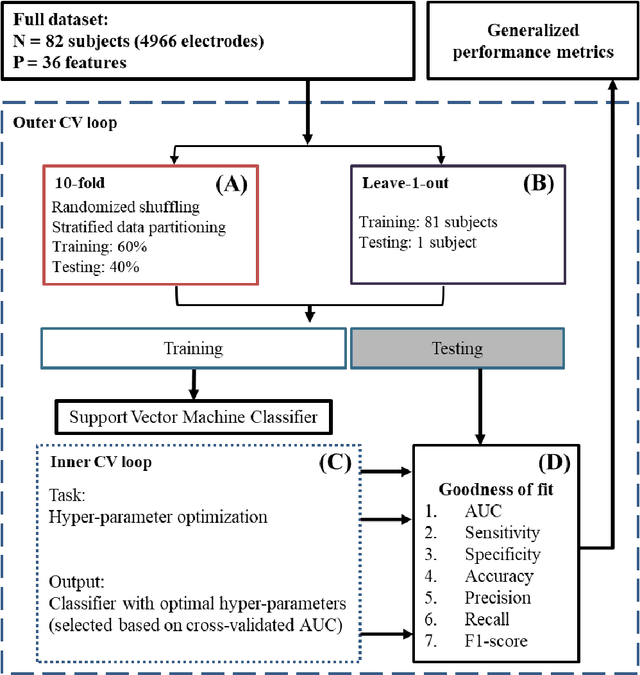
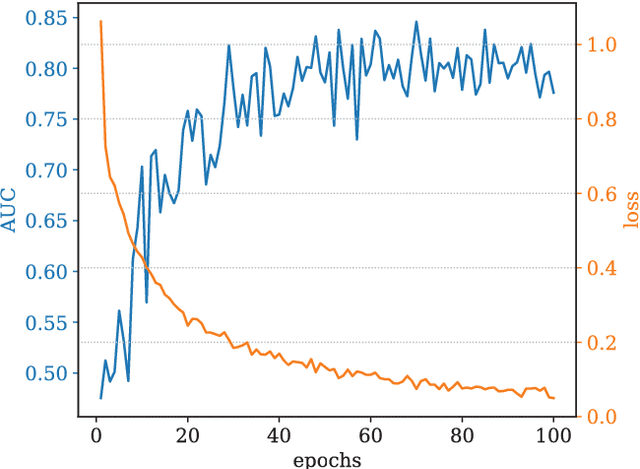
Abstract:An ability to map seizure-generating brain tissue, i.e., the seizure onset zone (SOZ), without recording actual seizures could reduce the duration of invasive EEG monitoring for patients with drug-resistant epilepsy. A widely-adopted practice in the literature is to compare the incidence (events/time) of putative pathological electrophysiological biomarkers associated with epileptic brain tissue with the SOZ determined from spontaneous seizures recorded with intracranial EEG, primarily using a single biomarker. Clinical translation of the previous efforts suffers from their inability to generalize across multiple patients because of (a) the inter-patient variability and (b) the temporal variability in the epileptogenic activity. Here, we report an artificial intelligence-based approach for combining multiple interictal electrophysiological biomarkers and their temporal characteristics as a way of accounting for the above barriers and show that it can reliably identify seizure onset zones in a study cohort of 82 patients who underwent evaluation for drug-resistant epilepsy. Our investigation provides evidence that utilizing the complementary information provided by multiple electrophysiological biomarkers and their temporal characteristics can significantly improve the localization potential compared to previously published single-biomarker incidence-based approaches, resulting in an average area under ROC curve (AUC) value of 0.73 in a cohort of 82 patients. Our results also suggest that recording durations between ninety minutes and two hours are sufficient to localize SOZs with accuracies that may prove clinically relevant. The successful validation of our approach on a large cohort of 82 patients warrants future investigation on the feasibility of utilizing intra-operative EEG monitoring and artificial intelligence to localize epileptogenic brain tissue.
Convolutional Neural Networks for Epileptic Seizure Prediction
Nov 05, 2018
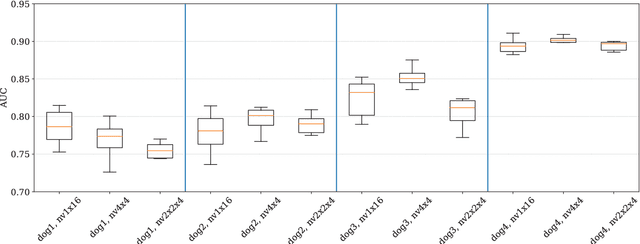
Abstract:Epilepsy is the most common neurological disorder and an accurate forecast of seizures would help to overcome the patient's uncertainty and helplessness. In this contribution, we present and discuss a novel methodology for the classification of intracranial electroencephalography (iEEG) for seizure prediction. Contrary to previous approaches, we categorically refrain from an extraction of hand-crafted features and use a convolutional neural network (CNN) topology instead for both the determination of suitable signal characteristics and the binary classification of preictal and interictal segments. Three different models have been evaluated on public datasets with long-term recordings from four dogs and three patients. Overall, our findings demonstrate the general applicability. In this work we discuss the strengths and limitations of our methodology.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge