Benedetta Bodini
Axial multi-layer perceptron architecture for automatic segmentation of choroid plexus in multiple sclerosis
Sep 08, 2021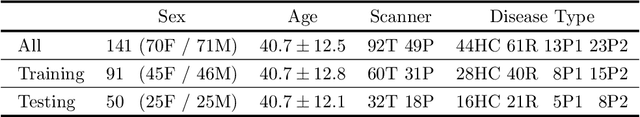
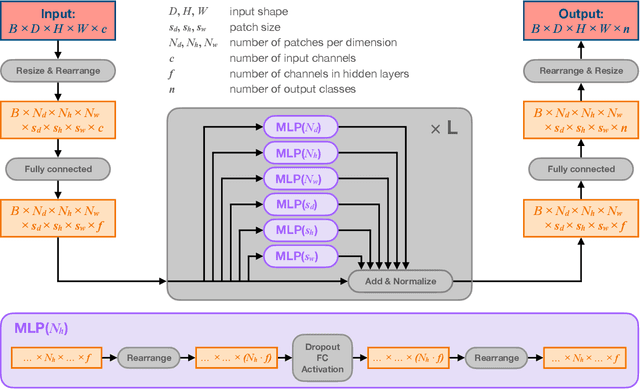
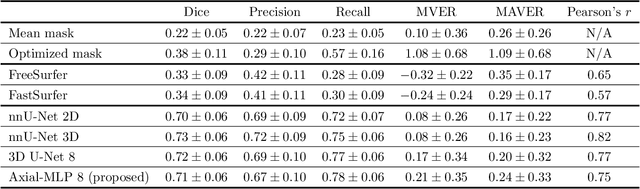
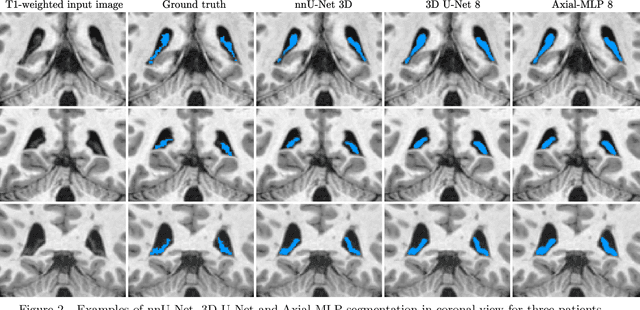
Abstract:Choroid plexuses (CP) are structures of the ventricles of the brain which produce most of the cerebrospinal fluid (CSF). Several postmortem and in vivo studies have pointed towards their role in the inflammatory process in multiple sclerosis (MS). Automatic segmentation of CP from MRI thus has high value for studying their characteristics in large cohorts of patients. To the best of our knowledge, the only freely available tool for CP segmentation is FreeSurfer but its accuracy for this specific structure is poor. In this paper, we propose to automatically segment CP from non-contrast enhanced T1-weighted MRI. To that end, we introduce a new model called "Axial-MLP" based on an assembly of Axial multi-layer perceptrons (MLPs). This is inspired by recent works which showed that the self-attention layers of Transformers can be replaced with MLPs. This approach is systematically compared with a standard 3D U-Net, nnU-Net, Freesurfer and FastSurfer. For our experiments, we make use of a dataset of 141 subjects (44 controls and 97 patients with MS). We show that all the tested deep learning (DL) methods outperform FreeSurfer (Dice around 0.7 for DL vs 0.33 for FreeSurfer). Axial-MLP is competitive with U-Nets even though it is slightly less accurate. The conclusions of our paper are two-fold: 1) the studied deep learning methods could be useful tools to study CP in large cohorts of MS patients; 2)~Axial-MLP is a potentially viable alternative to convolutional neural networks for such tasks, although it could benefit from further improvements.
Learning Myelin Content in Multiple Sclerosis from Multimodal MRI through Adversarial Training
Jun 08, 2018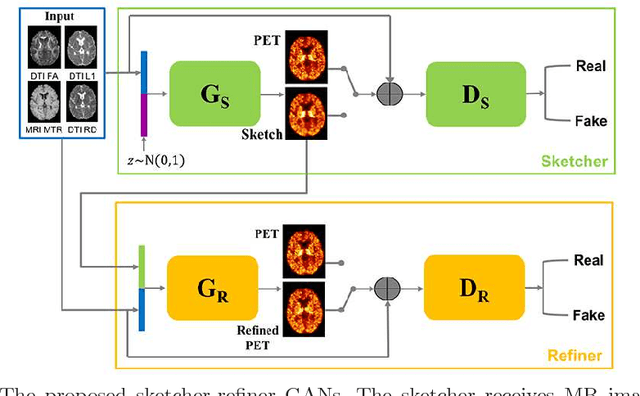
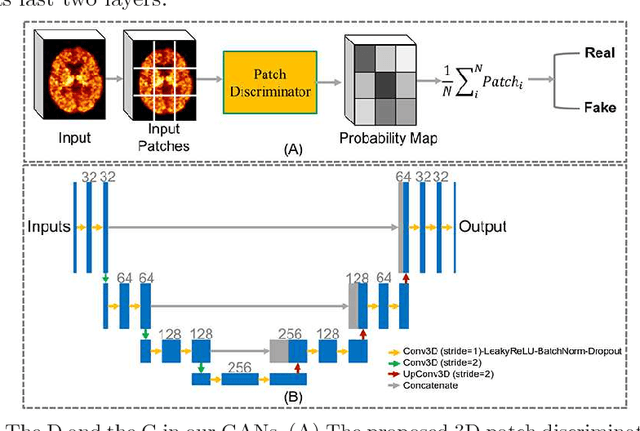
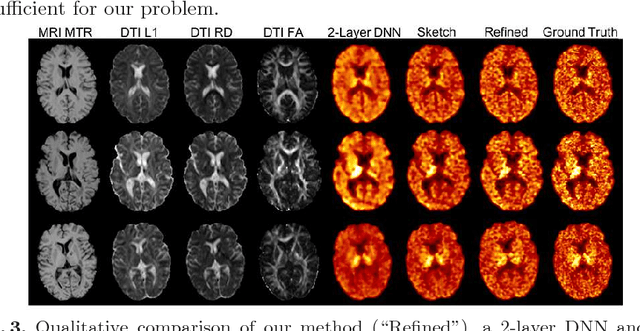
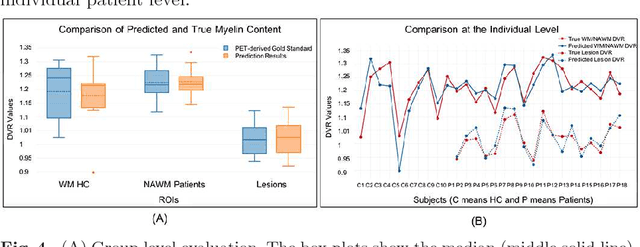
Abstract:Multiple sclerosis (MS) is a demyelinating disease of the central nervous system (CNS). A reliable measure of the tissue myelin content is therefore essential for the understanding of the physiopathology of MS, tracking progression and assessing treatment efficacy. Positron emission tomography (PET) with $[^{11} \mbox{C}] \mbox{PIB}$ has been proposed as a promising biomarker for measuring myelin content changes in-vivo in MS. However, PET imaging is expensive and invasive due to the injection of a radioactive tracer. On the contrary, magnetic resonance imaging (MRI) is a non-invasive, widely available technique, but existing MRI sequences do not provide, to date, a reliable, specific, or direct marker of either demyelination or remyelination. In this work, we therefore propose Sketcher-Refiner Generative Adversarial Networks (GANs) with specifically designed adversarial loss functions to predict the PET-derived myelin content map from a combination of MRI modalities. The prediction problem is solved by a sketch-refinement process in which the sketcher generates the preliminary anatomical and physiological information and the refiner refines and generates images reflecting the tissue myelin content in the human brain. We evaluated the ability of our method to predict myelin content at both global and voxel-wise levels. The evaluation results show that the demyelination in lesion regions and myelin content in normal-appearing white matter (NAWM) can be well predicted by our method. The method has the potential to become a useful tool for clinical management of patients with MS.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge