Anne Kerbrat
Monitoring morphometric drift in lifelong learning segmentation of the spinal cord
May 02, 2025Abstract:Morphometric measures derived from spinal cord segmentations can serve as diagnostic and prognostic biomarkers in neurological diseases and injuries affecting the spinal cord. While robust, automatic segmentation methods to a wide variety of contrasts and pathologies have been developed over the past few years, whether their predictions are stable as the model is updated using new datasets has not been assessed. This is particularly important for deriving normative values from healthy participants. In this study, we present a spinal cord segmentation model trained on a multisite $(n=75)$ dataset, including 9 different MRI contrasts and several spinal cord pathologies. We also introduce a lifelong learning framework to automatically monitor the morphometric drift as the model is updated using additional datasets. The framework is triggered by an automatic GitHub Actions workflow every time a new model is created, recording the morphometric values derived from the model's predictions over time. As a real-world application of the proposed framework, we employed the spinal cord segmentation model to update a recently-introduced normative database of healthy participants containing commonly used measures of spinal cord morphometry. Results showed that: (i) our model outperforms previous versions and pathology-specific models on challenging lumbar spinal cord cases, achieving an average Dice score of $0.95 \pm 0.03$; (ii) the automatic workflow for monitoring morphometric drift provides a quick feedback loop for developing future segmentation models; and (iii) the scaling factor required to update the database of morphometric measures is nearly constant among slices across the given vertebral levels, showing minimum drift between the current and previous versions of the model monitored by the framework. The model is freely available in Spinal Cord Toolbox v7.0.
Segmentation of Multiple Sclerosis Lesions across Hospitals: Learn Continually or Train from Scratch?
Oct 27, 2022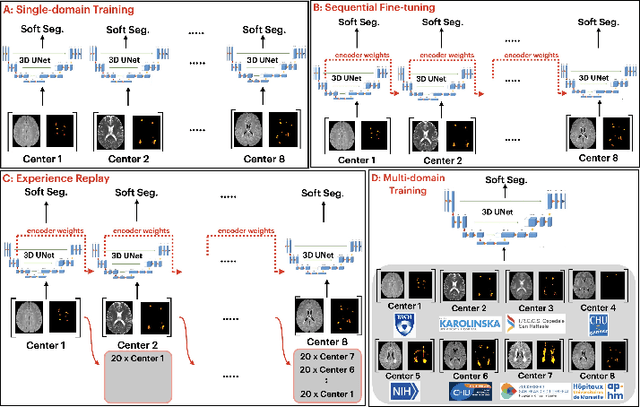
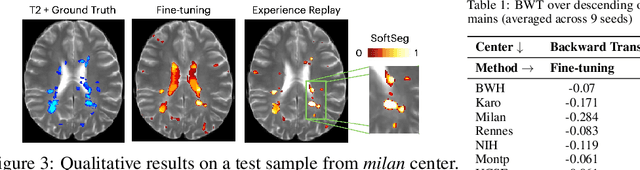
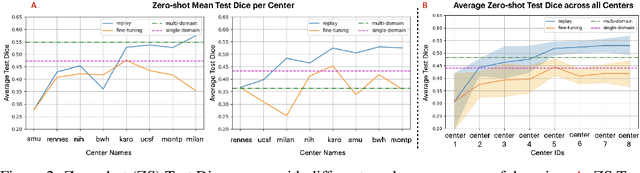
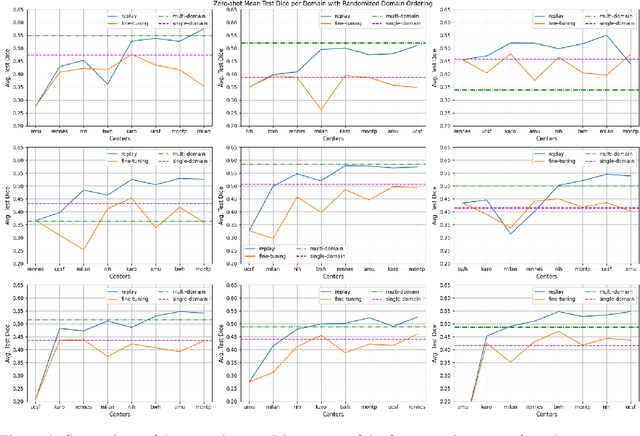
Abstract:Segmentation of Multiple Sclerosis (MS) lesions is a challenging problem. Several deep-learning-based methods have been proposed in recent years. However, most methods tend to be static, that is, a single model trained on a large, specialized dataset, which does not generalize well. Instead, the model should learn across datasets arriving sequentially from different hospitals by building upon the characteristics of lesions in a continual manner. In this regard, we explore experience replay, a well-known continual learning method, in the context of MS lesion segmentation across multi-contrast data from 8 different hospitals. Our experiments show that replay is able to achieve positive backward transfer and reduce catastrophic forgetting compared to sequential fine-tuning. Furthermore, replay outperforms the multi-domain training, thereby emerging as a promising solution for the segmentation of MS lesions. The code is available at this link: https://github.com/naga-karthik/continual-learning-ms
Effectiveness of regional diffusion MRI measures in distinguishing multiple sclerosis abnormalities within the cervical spinal cord
Aug 09, 2021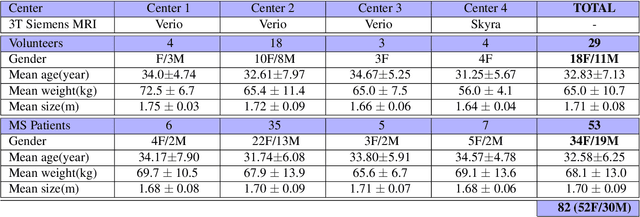



Abstract:Multiple sclerosis is an inflammatory disorder of the central nervous system. Quantitative MRI has huge potential to provide intrinsic and normative values of tissue properties useful for diagnosis, prognosis and ultimately clinical follow-up of this disease. However, there is a large discrepancy between the clinical observations and how the pathology is exhibited in MRI brain scans. Complementary to brain imaging, the study of multiple sclerosis lesions in the spinal cord has recently gained interest as a potential marker for early physical impairment. Therefore, investigating how the spinal cord is damaged using quantitative imaging, in particular, diffusion MRI, becomes an acute challenge. In this work, we extract average diffusion MRI metrics per vertebral level from spinal cord data acquired from multiple clinical sites. The diffusion-based metrics involved are extracted from the diffusion tensor imaging and Ball-and-Stick models and quantified for every cervical vertebral level using a collection of image processing methods and an atlas-based approach. Then, we perform a statistical analysis study to characterize the feasibility of these metrics to detect lesions. Specifically, we study the usefulness of combining different metrics to improve the accuracy prediction score associated with the presence of multiple sclerosis lesions. We demonstrate the grade of sensitivity to underlying microstructure changes in MS patients of each metric. Ball-and-Stick provides novel information about the MS damage to tissue microstructure. In addition, we show that choosing a subset of metrics: [FA, RD, MD] and [FWW, MD, Stick-AD, RD], which bring complementary information, has significantly increased the prediction score of the presence of the MS lesion in the cervical spinal cord.
Evaluation of distortion correction methods in diffusion MRI of the spinal cord
Aug 09, 2021



Abstract:Background: Magnetic field inhomogeneities generate important geometric distortions in reconstructed echo-planar images. Various procedures were proposed for correcting these distortions on brain images; yet, few neuroimaging studies tailored and incorporated the use of these techniques in spinal cord diffusion MRI. Purpose: We present a comparative evaluation of distortion correction methods that use the reversed gradient polarity technique on spinal cord. We propose novel geometric metrics to measure the alignment of the reconstructed diffusion model with the apparent centerline of the spinal cord. Subjects: 95 subjects, among which 29 healthy controls and 66 multiple sclerosis patients. Assessment: Geometric distortions were corrected using 4 state-of-the-art methods. We measured the alignment of the principal direction of diffusion with the apparent centerline of the spine after correction and the correlation with the reference anatomical image. Results are computed per vertebral level, to evaluate the impact on different portions of the spine. Besides, subjective evaluation of the quality of the correction of healthy subjects images was performed by three expert raters. Results: As a result of distortion correction, the diffusion directions are better aligned locally with the centerline, in particular at both ends of the acquisition window. The cross-correlation with anatomical image is also improved by Hyperelastic Susceptibility Artefact Correction (HySCO) and block-matching. The subjective evaluation for HySCO is significantly better (p < 0.05) than for Block-Matching; TOPUP performs significantly worse than the three other methods. Conclusion: Correction based on HySCO provide best results among the selected methods.
Reproducibility and Evolution of Diffusion MRI Measurements within the Cervical Spinal Cord in Multiple Sclerosis
Aug 08, 2021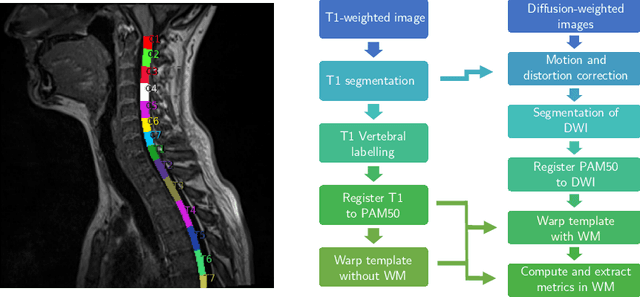

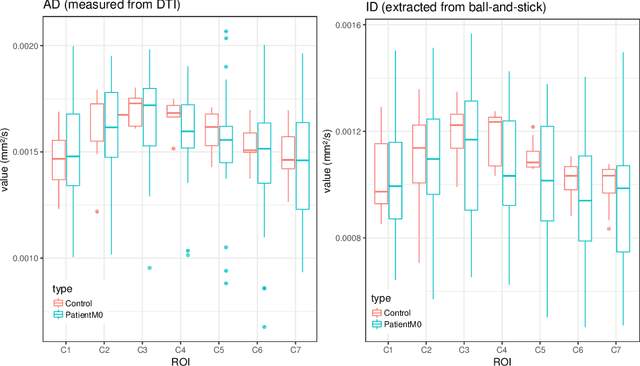

Abstract:In Multiple Sclerosis (MS), there is a large discrepancy between the clinical observations and how the pathology is exhibited on brain images, this is known as the clinical-radiological paradox (CRP). One of the hypotheses is that the clinical deficit may be more related to the spinal cord damage than the number or location of lesions in the brain. Therefore, investigating how the spinal cord is damaged becomes an acute challenge to better understand and overcome the CRP. Diffusion MRI is known to provide quantitative figures of neuronal degeneration and axonal loss, in the brain as well as in the spinal cord. In this paper, we propose to investigate how diffusion MRI metrics vary in the different cervical regions with the progression of the disease. We first study the reproducibility of diffusion MRI on healthy volunteers with a test-retest procedure using both standard diffusion tensor imaging (DTI) and multi-compartment Ball-and-Stick models. Then, based on the test re-test quantitative calibration, we provide quantitative figures of pathology evolution between M0 and M12 in the cervical spine on a set of 31 MS patients, exhibiting how the pathology damage spans in the cervical spinal cord.
Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks
Sep 11, 2018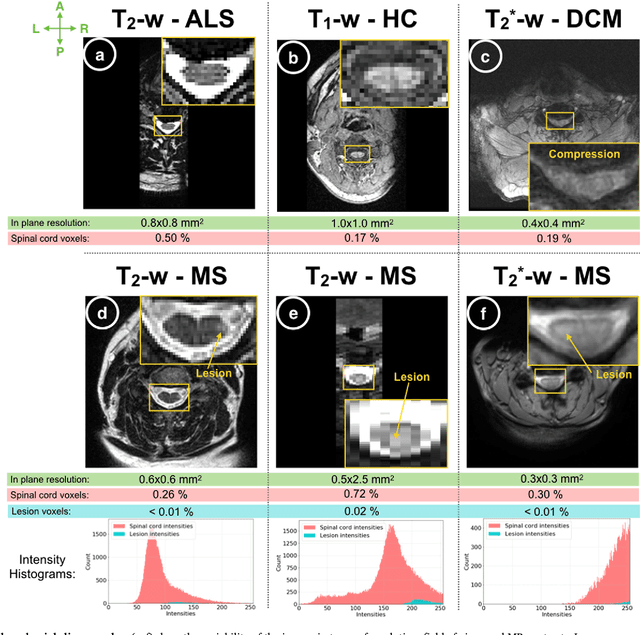
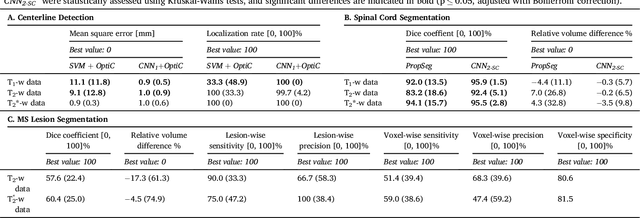
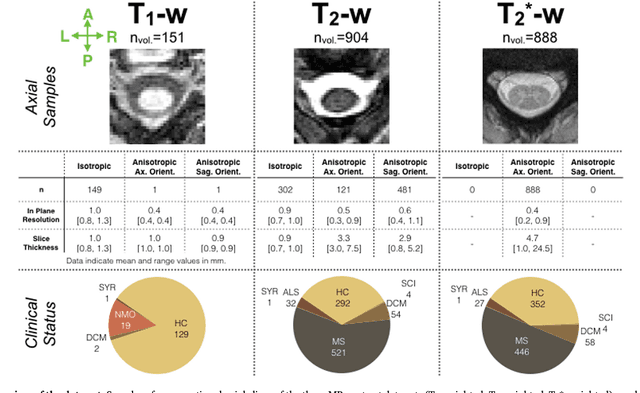
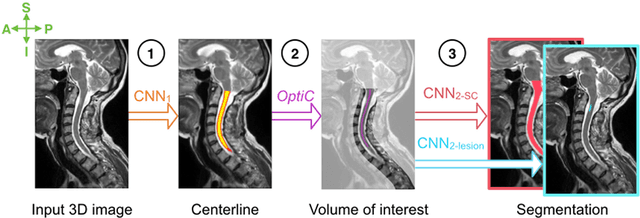
Abstract:The spinal cord is frequently affected by atrophy and/or lesions in multiple sclerosis (MS) patients. Segmentation of the spinal cord and lesions from MRI data provides measures of damage, which are key criteria for the diagnosis, prognosis, and longitudinal monitoring in MS. Automating this operation eliminates inter-rater variability and increases the efficiency of large-throughput analysis pipelines. Robust and reliable segmentation across multi-site spinal cord data is challenging because of the large variability related to acquisition parameters and image artifacts. The goal of this study was to develop a fully-automatic framework, robust to variability in both image parameters and clinical condition, for segmentation of the spinal cord and intramedullary MS lesions from conventional MRI data. Scans of 1,042 subjects (459 healthy controls, 471 MS patients, and 112 with other spinal pathologies) were included in this multi-site study (n=30). Data spanned three contrasts (T1-, T2-, and T2*-weighted) for a total of 1,943 volumes. The proposed cord and lesion automatic segmentation approach is based on a sequence of two Convolutional Neural Networks (CNNs). To deal with the very small proportion of spinal cord and/or lesion voxels compared to the rest of the volume, a first CNN with 2D dilated convolutions detects the spinal cord centerline, followed by a second CNN with 3D convolutions that segments the spinal cord and/or lesions. When compared against manual segmentation, our CNN-based approach showed a median Dice of 95% vs. 88% for PropSeg, a state-of-the-art spinal cord segmentation method. Regarding lesion segmentation on MS data, our framework provided a Dice of 60%, a relative volume difference of -15%, and a lesion-wise detection sensitivity and precision of 83% and 77%, respectively. The proposed framework is open-source and readily available in the Spinal Cord Toolbox.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge