Benjamin De Leener
Impact of Initialization on Intra-subject Pediatric Brain MR Image Registration: A Comparative Analysis between SyN ANTs and Deep Learning-Based Approaches
Jun 28, 2024



Abstract:This study evaluates the performance of conventional SyN ANTs and learning-based registration methods in the context of pediatric neuroimaging, specifically focusing on intrasubject deformable registration. The comparison involves three approaches: without (NR), with rigid (RR), and with rigid and affine (RAR) initializations. In addition to initialization, performances are evaluated in terms of accuracy, speed, and the impact of age intervals and sex per pair. Data consists of the publicly available MRI scans from the Calgary Preschool dataset, which includes 63 children aged 2-7 years, allowing for 431 registration pairs. We implemented the unsupervised DL framework with a U-Net architecture using DeepReg and it was 5-fold cross-validated. Evaluation includes Dice scores for tissue segmentation from 18 smaller regions obtained by SynthSeg, analysis of log Jacobian determinants, and registration pro-rated training and inference times. Learning-based approaches, with or without linear initializations, exhibit slight superiority over SyN ANTs in terms of Dice scores. Indeed, DL-based implementations with RR and RAR initializations significantly outperform SyN ANTs. Both SyN ANTs and DL-based registration involve parameter optimization, but the choice between these methods depends on the scale of registration: network-based for broader coverage or SyN ANTs for specific structures. Both methods face challenges with larger age intervals due to greater growth changes. The main takeaway is that while DL-based methods show promise with faster and more accurate registrations, SyN ANTs remains robust and generalizable without the need for extensive training, highlighting the importance of method selection based on specific registration needs in the pediatric context. Our code is available at https://github.com/neuropoly/pediatric-DL-registration
* Accepted for publication at the Journal of Machine Learning for Biomedical Imaging (MELBA) https://melba-journal.org/2024:013
Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks
Sep 11, 2018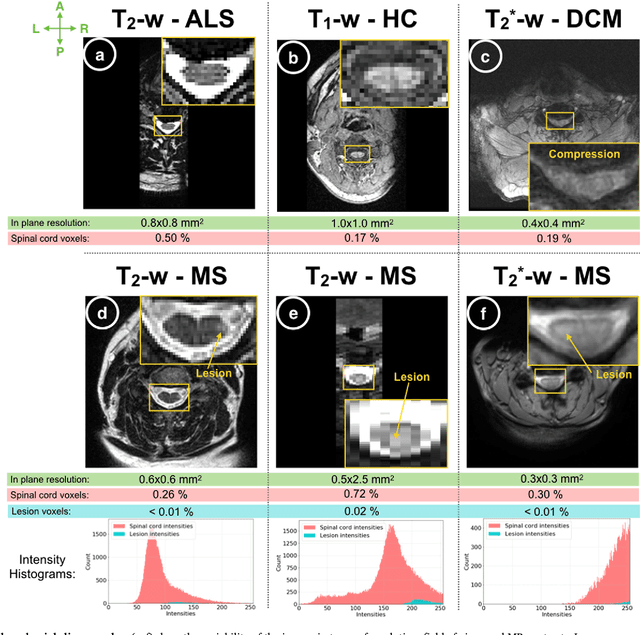
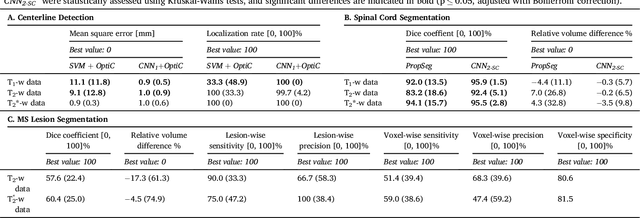
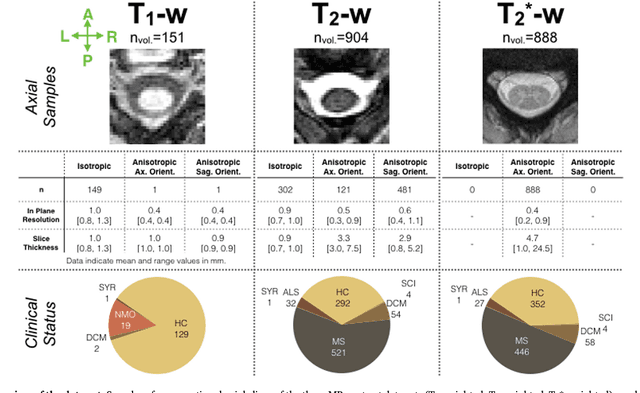
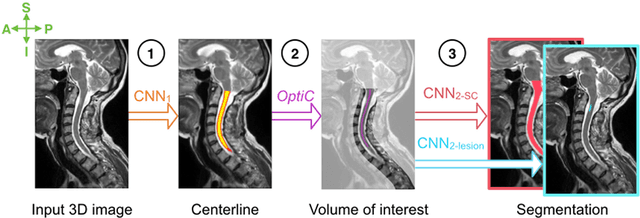
Abstract:The spinal cord is frequently affected by atrophy and/or lesions in multiple sclerosis (MS) patients. Segmentation of the spinal cord and lesions from MRI data provides measures of damage, which are key criteria for the diagnosis, prognosis, and longitudinal monitoring in MS. Automating this operation eliminates inter-rater variability and increases the efficiency of large-throughput analysis pipelines. Robust and reliable segmentation across multi-site spinal cord data is challenging because of the large variability related to acquisition parameters and image artifacts. The goal of this study was to develop a fully-automatic framework, robust to variability in both image parameters and clinical condition, for segmentation of the spinal cord and intramedullary MS lesions from conventional MRI data. Scans of 1,042 subjects (459 healthy controls, 471 MS patients, and 112 with other spinal pathologies) were included in this multi-site study (n=30). Data spanned three contrasts (T1-, T2-, and T2*-weighted) for a total of 1,943 volumes. The proposed cord and lesion automatic segmentation approach is based on a sequence of two Convolutional Neural Networks (CNNs). To deal with the very small proportion of spinal cord and/or lesion voxels compared to the rest of the volume, a first CNN with 2D dilated convolutions detects the spinal cord centerline, followed by a second CNN with 3D convolutions that segments the spinal cord and/or lesions. When compared against manual segmentation, our CNN-based approach showed a median Dice of 95% vs. 88% for PropSeg, a state-of-the-art spinal cord segmentation method. Regarding lesion segmentation on MS data, our framework provided a Dice of 60%, a relative volume difference of -15%, and a lesion-wise detection sensitivity and precision of 83% and 77%, respectively. The proposed framework is open-source and readily available in the Spinal Cord Toolbox.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge