Adam Martersteck
Interpretation of Brain Morphology in Association to Alzheimer's Disease Dementia Classification Using Graph Convolutional Networks on Triangulated Meshes
Aug 20, 2020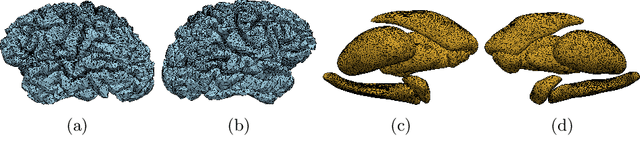
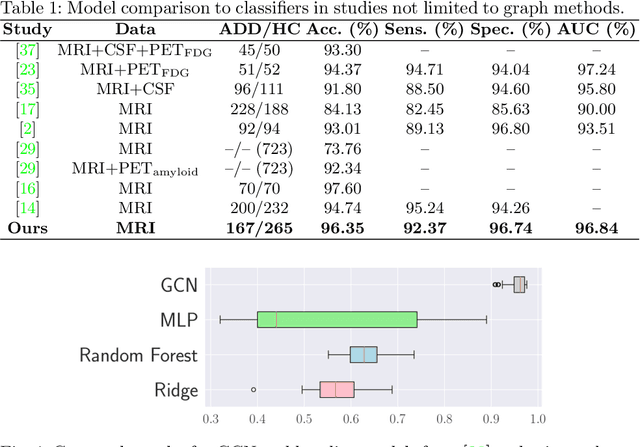
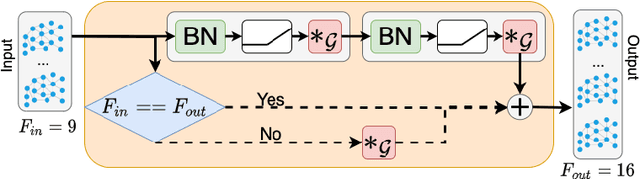
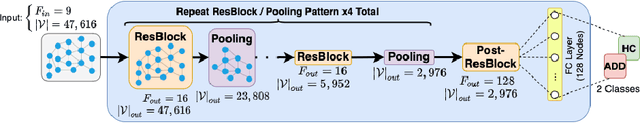
Abstract:We propose a mesh-based technique to aid in the classification of Alzheimer's disease dementia (ADD) using mesh representations of the cortex and subcortical structures. Deep learning methods for classification tasks that utilize structural neuroimaging often require extensive learning parameters to optimize. Frequently, these approaches for automated medical diagnosis also lack visual interpretability for areas in the brain involved in making a diagnosis. This work: (a) analyzes brain shape using surface information of the cortex and subcortical structures, (b) proposes a residual learning framework for state-of-the-art graph convolutional networks which offer a significant reduction in learnable parameters, and (c) offers visual interpretability of the network via class-specific gradient information that localizes important regions of interest in our inputs. With our proposed method leveraging the use of cortical and subcortical surface information, we outperform other machine learning methods with a 96.35% testing accuracy for the ADD vs. healthy control problem. We confirm the validity of our model by observing its performance in a 25-trial Monte Carlo cross-validation. The generated visualization maps in our study show correspondences with current knowledge regarding the structural localization of pathological changes in the brain associated to dementia of the Alzheimer's type.
Neuroimaging Modality Fusion in Alzheimer's Classification Using Convolutional Neural Networks
Nov 13, 2018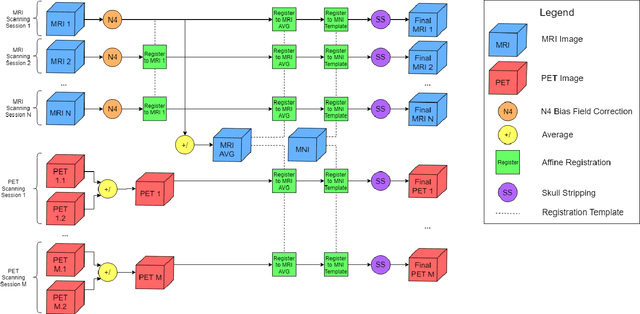
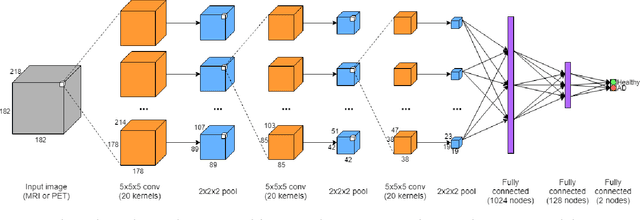
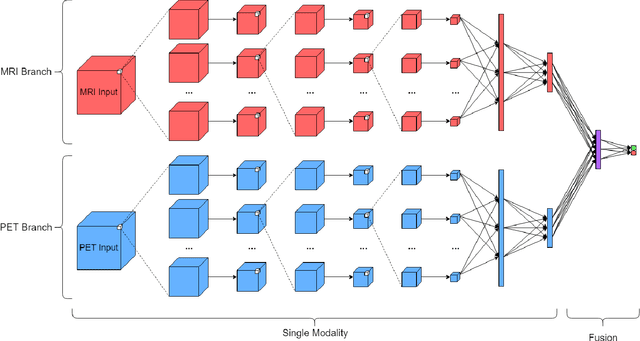
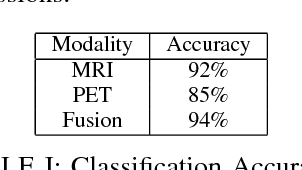
Abstract:Automated methods for Alzheimer's disease (AD) classification have the potential for great clinical benefits and may provide insight for combating the disease. Machine learning, and more specifically deep neural networks, have been shown to have great efficacy in this domain. These algorithms often use neurological imaging data such as MRI and PET, but a comprehensive and balanced comparison of these modalities has not been performed. In order to accurately determine the relative strength of each imaging variant, this work performs a comparison study in the context of Alzheimer's dementia classification using the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset. Furthermore, this work analyzes the benefits of using both modalities in a fusion setting and discusses how these data types may be leveraged in future AD studies using deep learning.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge