Unlocking Real-Time Fluorescence Lifetime Imaging: Multi-Pixel Parallelism for FPGA-Accelerated Processing
Paper and Code
Oct 09, 2024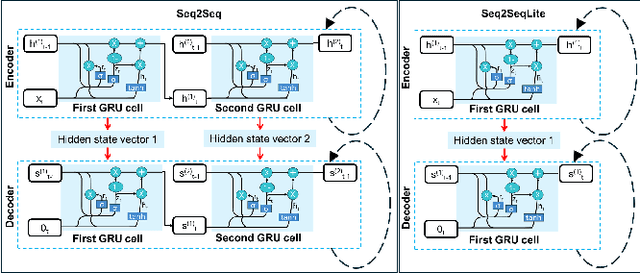
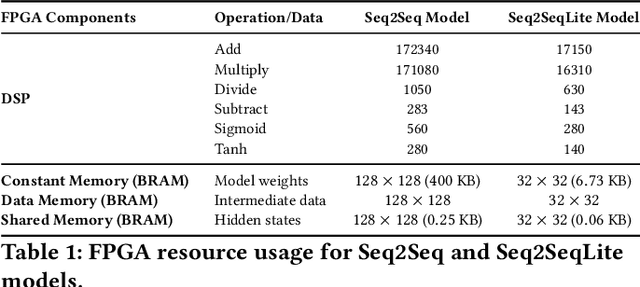

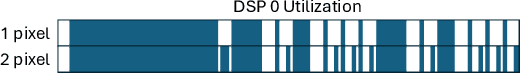
Fluorescence lifetime imaging (FLI) is a widely used technique in the biomedical field for measuring the decay times of fluorescent molecules, providing insights into metabolic states, protein interactions, and ligand-receptor bindings. However, its broader application in fast biological processes, such as dynamic activity monitoring, and clinical use, such as in guided surgery, is limited by long data acquisition times and computationally demanding data processing. While deep learning has reduced post-processing times, time-resolved data acquisition remains a bottleneck for real-time applications. To address this, we propose a method to achieve real-time FLI using an FPGA-based hardware accelerator. Specifically, we implemented a GRU-based sequence-to-sequence (Seq2Seq) model on an FPGA board compatible with time-resolved cameras. The GRU model balances accurate processing with the resource constraints of FPGAs, which have limited DSP units and BRAM. The limited memory and computational resources on the FPGA require efficient scheduling of operations and memory allocation to deploy deep learning models for low-latency applications. We address these challenges by using STOMP, a queue-based discrete-event simulator that automates and optimizes task scheduling and memory management on hardware. By integrating a GRU-based Seq2Seq model and its compressed version, called Seq2SeqLite, generated through knowledge distillation, we were able to process multiple pixels in parallel, reducing latency compared to sequential processing. We explore various levels of parallelism to achieve an optimal balance between performance and resource utilization. Our results indicate that the proposed techniques achieved a 17.7x and 52.0x speedup over manual scheduling for the Seq2Seq model and the Seq2SeqLite model, respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge