Retinal Image Segmentation with Small Datasets
Paper and Code
Mar 09, 2023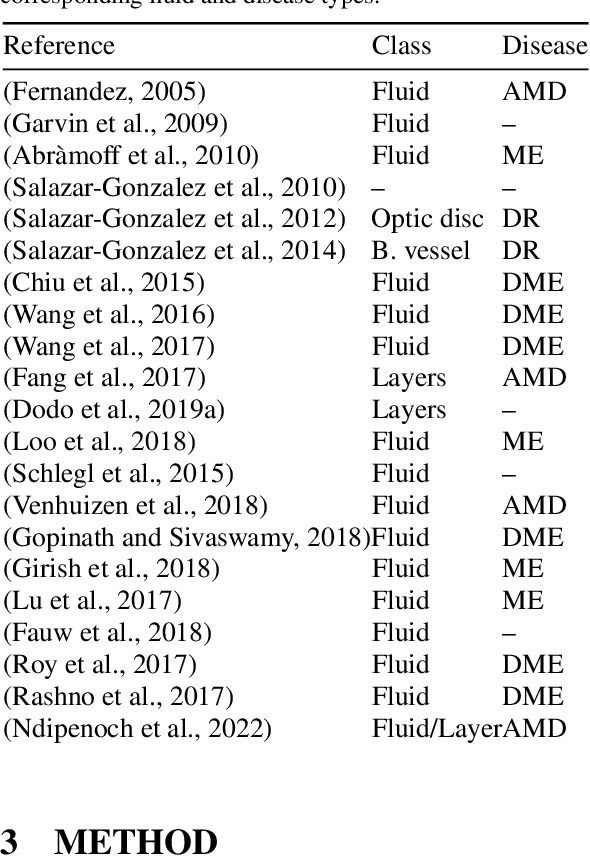
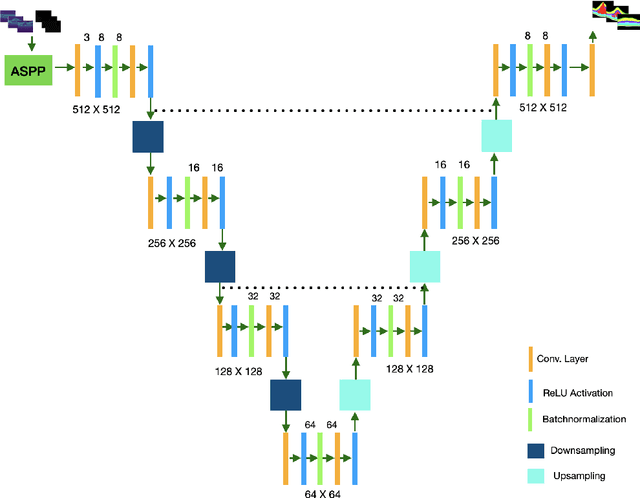
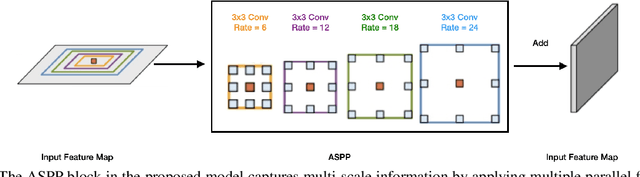
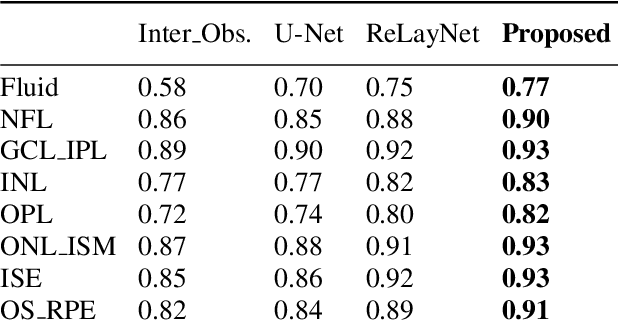
Many eye diseases like Diabetic Macular Edema (DME), Age-related Macular Degeneration (AMD), and Glaucoma manifest in the retina, can cause irreversible blindness or severely impair the central version. The Optical Coherence Tomography (OCT), a 3D scan of the retina with high qualitative information about the retinal morphology, can be used to diagnose and monitor changes in the retinal anatomy. Many Deep Learning (DL) methods have shared the success of developing an automated tool to monitor pathological changes in the retina. However, the success of these methods depend mainly on large datasets. To address the challenge from very small and limited datasets, we proposed a DL architecture termed CoNet (Coherent Network) for joint segmentation of layers and fluids in retinal OCT images on very small datasets (less than a hundred training samples). The proposed model was evaluated on the publicly available Duke DME dataset consisting of 110 B-Scans from 10 patients suffering from DME. Experimental results show that the proposed model outperformed both the human experts' annotation and the current state-of-the-art architectures by a clear margin with a mean Dice Score of 88% when trained on 55 images without any data augmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge