MG-NET: Leveraging Pseudo-Imaging for Multi-Modal Metagenome Analysis
Paper and Code
Jul 21, 2021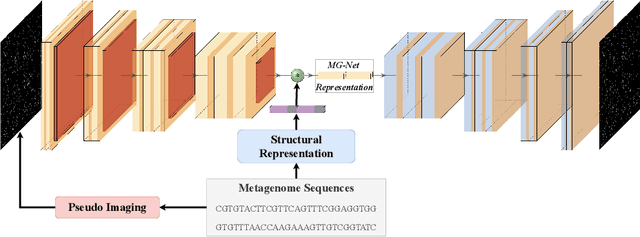
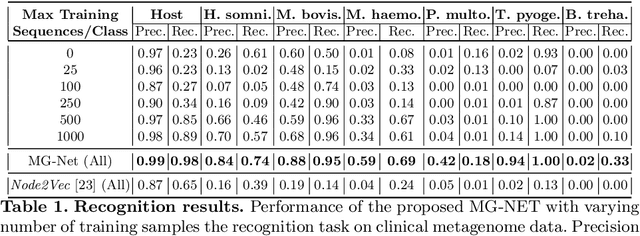
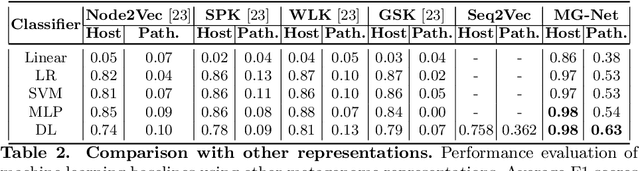
The emergence of novel pathogens and zoonotic diseases like the SARS-CoV-2 have underlined the need for developing novel diagnosis and intervention pipelines that can learn rapidly from small amounts of labeled data. Combined with technological advances in next-generation sequencing, metagenome-based diagnostic tools hold much promise to revolutionize rapid point-of-care diagnosis. However, there are significant challenges in developing such an approach, the chief among which is to learn self-supervised representations that can help detect novel pathogen signatures with very low amounts of labeled data. This is particularly a difficult task given that closely related pathogens can share more than 90% of their genome structure. In this work, we address these challenges by proposing MG-Net, a self-supervised representation learning framework that leverages multi-modal context using pseudo-imaging data derived from clinical metagenome sequences. We show that the proposed framework can learn robust representations from unlabeled data that can be used for downstream tasks such as metagenome sequence classification with limited access to labeled data. Extensive experiments show that the learned features outperform current baseline metagenome representations, given only 1000 samples per class.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge