Antigen-Specific Antibody Design via Direct Energy-based Preference Optimization
Paper and Code
Mar 25, 2024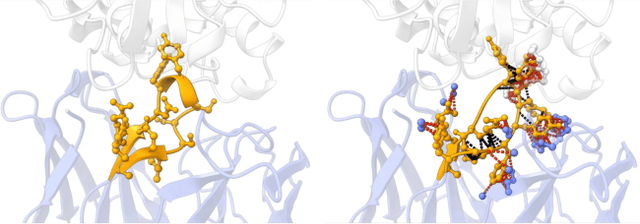
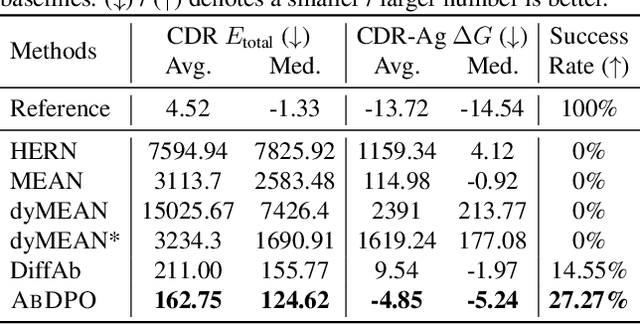
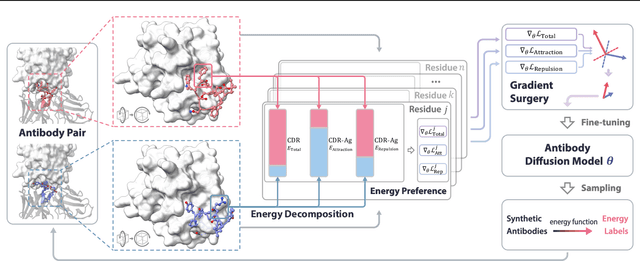
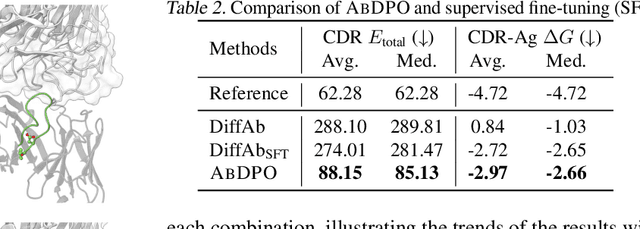
Antibody design, a crucial task with significant implications across various disciplines such as therapeutics and biology, presents considerable challenges due to its intricate nature. In this paper, we tackle antigen-specific antibody design as a protein sequence-structure co-design problem, considering both rationality and functionality. Leveraging a pre-trained conditional diffusion model that jointly models sequences and structures of complementarity-determining regions (CDR) in antibodies with equivariant neural networks, we propose direct energy-based preference optimization to guide the generation of antibodies with both rational structures and considerable binding affinities to given antigens. Our method involves fine-tuning the pre-trained diffusion model using a residue-level decomposed energy preference. Additionally, we employ gradient surgery to address conflicts between various types of energy, such as attraction and repulsion. Experiments on RAbD benchmark show that our approach effectively optimizes the energy of generated antibodies and achieves state-of-the-art performance in designing high-quality antibodies with low total energy and high binding affinity, demonstrating the superiority of our approach.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge