Yunsung Chung
Weighted Temporal Decay Loss for Learning Wearable PPG Data with Sparse Clinical Labels
Feb 02, 2026Abstract:Advances in wearable computing and AI have increased interest in leveraging PPG for health monitoring over the past decade. One of the biggest challenges in developing health algorithms based on such biosignals is the sparsity of clinical labels, which makes biosignals temporally distant from lab draws less reliable for supervision. To address this problem, we introduce a simple training strategy that learns a biomarker-specific decay of sample weight over the time gap between a segment and its ground truth label and uses this weight in the loss with a regularizer to prevent trivial solutions. On smartwatch PPG from 450 participants across 10 biomarkers, the approach improves over baselines. In the subject-wise setting, the proposed approach averages 0.715 AUPRC, compared to 0.674 for a fine-tuned self-supervised baseline and 0.626 for a feature-based Random Forest. A comparison of four decay families shows that a simple linear decay function is most robust on average. Beyond accuracy, the learned decay rates summarize how quickly each biomarker's PPG evidence becomes stale, providing an interpretable view of temporal sensitivity.
SOFA: Deep Learning Framework for Simulating and Optimizing Atrial Fibrillation Ablation
Aug 11, 2025



Abstract:Atrial fibrillation (AF) is a prevalent cardiac arrhythmia often treated with catheter ablation procedures, but procedural outcomes are highly variable. Evaluating and improving ablation efficacy is challenging due to the complex interaction between patient-specific tissue and procedural factors. This paper asks two questions: Can AF recurrence be predicted by simulating the effects of procedural parameters? How should we ablate to reduce AF recurrence? We propose SOFA (Simulating and Optimizing Atrial Fibrillation Ablation), a novel deep-learning framework that addresses these questions. SOFA first simulates the outcome of an ablation strategy by generating a post-ablation image depicting scar formation, conditioned on a patient's pre-ablation LGE-MRI and the specific procedural parameters used (e.g., ablation locations, duration, temperature, power, and force). During this simulation, it predicts AF recurrence risk. Critically, SOFA then introduces an optimization scheme that refines these procedural parameters to minimize the predicted risk. Our method leverages a multi-modal, multi-view generator that processes 2.5D representations of the atrium. Quantitative evaluations show that SOFA accurately synthesizes post-ablation images and that our optimization scheme leads to a 22.18\% reduction in the model-predicted recurrence risk. To the best of our knowledge, SOFA is the first framework to integrate the simulation of procedural effects, recurrence prediction, and parameter optimization, offering a novel tool for personalizing AF ablation.
SoK: Can Synthetic Images Replace Real Data? A Survey of Utility and Privacy of Synthetic Image Generation
Jun 24, 2025Abstract:Advances in generative models have transformed the field of synthetic image generation for privacy-preserving data synthesis (PPDS). However, the field lacks a comprehensive survey and comparison of synthetic image generation methods across diverse settings. In particular, when we generate synthetic images for the purpose of training a classifier, there is a pipeline of generation-sampling-classification which takes private training as input and outputs the final classifier of interest. In this survey, we systematically categorize existing image synthesis methods, privacy attacks, and mitigations along this generation-sampling-classification pipeline. To empirically compare diverse synthesis approaches, we provide a benchmark with representative generative methods and use model-agnostic membership inference attacks (MIAs) as a measure of privacy risk. Through this study, we seek to answer critical questions in PPDS: Can synthetic data effectively replace real data? Which release strategy balances utility and privacy? Do mitigations improve the utility-privacy tradeoff? Which generative models perform best across different scenarios? With a systematic evaluation of diverse methods, our study provides actionable insights into the utility-privacy tradeoffs of synthetic data generation methods and guides the decision on optimal data releasing strategies for real-world applications.
From Majority to Minority: A Diffusion-based Augmentation for Underrepresented Groups in Skin Lesion Analysis
Jun 26, 2024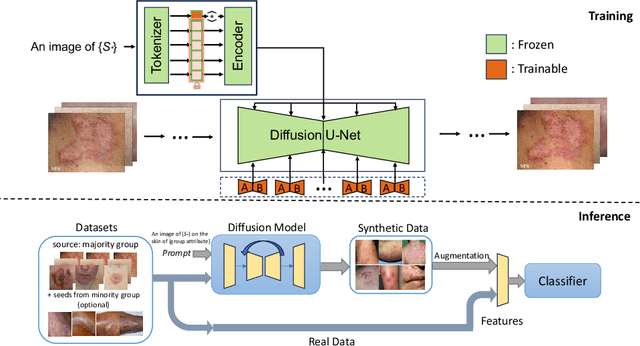
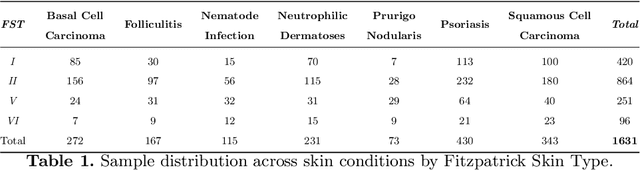

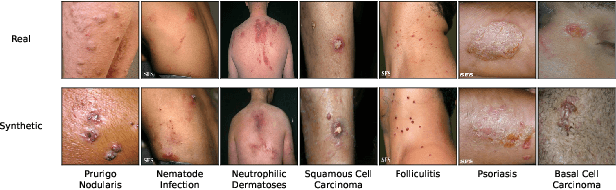
Abstract:AI-based diagnoses have demonstrated dermatologist-level performance in classifying skin cancer. However, such systems are prone to under-performing when tested on data from minority groups that lack sufficient representation in the training sets. Although data collection and annotation offer the best means for promoting minority groups, these processes are costly and time-consuming. Prior works have suggested that data from majority groups may serve as a valuable information source to supplement the training of diagnosis tools for minority groups. In this work, we propose an effective diffusion-based augmentation framework that maximizes the use of rich information from majority groups to benefit minority groups. Using groups with different skin types as a case study, our results show that the proposed framework can generate synthetic images that improve diagnostic results for the minority groups, even when there is little or no reference data from these target groups. The practical value of our work is evident in medical imaging analysis, where under-diagnosis persists as a problem for certain groups due to insufficient representation.
FBA-Net: Foreground and Background Aware Contrastive Learning for Semi-Supervised Atrium Segmentation
Jun 27, 2023Abstract:Medical image segmentation of gadolinium enhancement magnetic resonance imaging (GE MRI) is an important task in clinical applications. However, manual annotation is time-consuming and requires specialized expertise. Semi-supervised segmentation methods that leverage both labeled and unlabeled data have shown promise, with contrastive learning emerging as a particularly effective approach. In this paper, we propose a contrastive learning strategy of foreground and background representations for semi-supervised 3D medical image segmentation (FBA-Net). Specifically, we leverage the contrastive loss to learn representations of both the foreground and background regions in the images. By training the network to distinguish between foreground-background pairs, we aim to learn a representation that can effectively capture the anatomical structures of interest. Experiments on three medical segmentation datasets demonstrate state-of-the-art performance. Notably, our method achieves a Dice score of 91.31% with only 20% labeled data, which is remarkably close to the 91.62% score of the fully supervised method that uses 100% labeled data on the left atrium dataset. Our framework has the potential to advance the field of semi-supervised 3D medical image segmentation and enable more efficient and accurate analysis of medical images with a limited amount of annotated labels.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge