Yannik Glaser
Deep Learning Enables Large-Scale Shape and Appearance Modeling in Total-Body DXA Imaging
Aug 13, 2025Abstract:Total-body dual X-ray absorptiometry (TBDXA) imaging is a relatively low-cost whole-body imaging modality, widely used for body composition assessment. We develop and validate a deep learning method for automatic fiducial point placement on TBDXA scans using 1,683 manually-annotated TBDXA scans. The method achieves 99.5% percentage correct keypoints in an external testing dataset. To demonstrate the value for shape and appearance modeling (SAM), our method is used to place keypoints on 35,928 scans for five different TBDXA imaging modes, then associations with health markers are tested in two cohorts not used for SAM model generation using two-sample Kolmogorov-Smirnov tests. SAM feature distributions associated with health biomarkers are shown to corroborate existing evidence and generate new hypotheses on body composition and shape's relationship to various frailty, metabolic, inflammation, and cardiometabolic health markers. Evaluation scripts, model weights, automatic point file generation code, and triangulation files are available at https://github.com/hawaii-ai/dxa-pointplacement.
Artificial Intelligence-Informed Handheld Breast Ultrasound for Screening: A Systematic Review of Diagnostic Test Accuracy
Nov 11, 2024



Abstract:Background. Breast cancer screening programs using mammography have led to significant mortality reduction in high-income countries. However, many low- and middle-income countries lack resources for mammographic screening. Handheld breast ultrasound (BUS) is a low-cost alternative but requires substantial training. Artificial intelligence (AI) enabled BUS may aid in both the detection (perception) and classification (interpretation) of breast cancer. Materials and Methods. This review (CRD42023493053) is reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) and SWiM (Synthesis Without Meta-analysis) guidelines. PubMed and Google Scholar were searched from January 1, 2016 to December 12, 2023. A meta-analysis was not attempted. Studies are grouped according to their AI task type, application time, and AI task. Study quality is assessed using the QUality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. Results. Of 763 candidate studies, 314 total full texts were reviewed. 34 studies are included. The AI tasks of included studies are as follows: 1 frame selection, 6 detection, 11 segmentation, and 16 classification. In total, 5.7 million BUS images from over 185,000 patients were used for AI training or validation. A single study included a prospective testing set. 79% of studies were at high or unclear risk of bias. Conclusion. There has been encouraging development of AI for BUS. Despite studies demonstrating high performance across all identified tasks, the evidence supporting AI-enhanced BUS generally lacks robustness. High-quality model validation will be key to realizing the potential for AI-enhanced BUS in increasing access to screening in resource-limited environments.
Learning a Clinically-Relevant Concept Bottleneck for Lesion Detection in Breast Ultrasound
Jun 29, 2024Abstract:Detecting and classifying lesions in breast ultrasound images is a promising application of artificial intelligence (AI) for reducing the burden of cancer in regions with limited access to mammography. Such AI systems are more likely to be useful in a clinical setting if their predictions can be explained to a radiologist. This work proposes an explainable AI model that provides interpretable predictions using a standard lexicon from the American College of Radiology's Breast Imaging and Reporting Data System (BI-RADS). The model is a deep neural network featuring a concept bottleneck layer in which known BI-RADS features are predicted before making a final cancer classification. This enables radiologists to easily review the predictions of the AI system and potentially fix errors in real time by modifying the concept predictions. In experiments, a model is developed on 8,854 images from 994 women with expert annotations and histological cancer labels. The model outperforms state-of-the-art lesion detection frameworks with 48.9 average precision on the held-out testing set, and for cancer classification, concept intervention is shown to increase performance from 0.876 to 0.885 area under the receiver operating characteristic curve. Training and evaluation code is available at https://github.com/hawaii-ai/bus-cbm.
WV-Net: A foundation model for SAR WV-mode satellite imagery trained using contrastive self-supervised learning on 10 million images
Jun 26, 2024
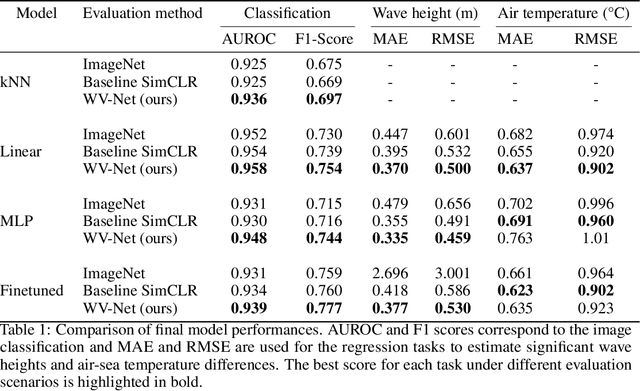

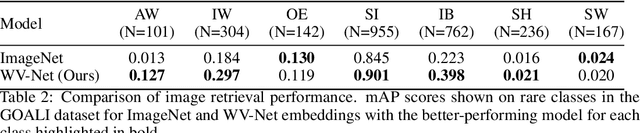
Abstract:The European Space Agency's Copernicus Sentinel-1 (S-1) mission is a constellation of C-band synthetic aperture radar (SAR) satellites that provide unprecedented monitoring of the world's oceans. S-1's wave mode (WV) captures 20x20 km image patches at 5 m pixel resolution and is unaffected by cloud cover or time-of-day. The mission's open data policy has made SAR data easily accessible for a range of applications, but the need for manual image annotations is a bottleneck that hinders the use of machine learning methods. This study uses nearly 10 million WV-mode images and contrastive self-supervised learning to train a semantic embedding model called WV-Net. In multiple downstream tasks, WV-Net outperforms a comparable model that was pre-trained on natural images (ImageNet) with supervised learning. Experiments show improvements for estimating wave height (0.50 vs 0.60 RMSE using linear probing), estimating near-surface air temperature (0.90 vs 0.97 RMSE), and performing multilabel-classification of geophysical and atmospheric phenomena (0.96 vs 0.95 micro-averaged AUROC). WV-Net embeddings are also superior in an unsupervised image-retrieval task and scale better in data-sparse settings. Together, these results demonstrate that WV-Net embeddings can support geophysical research by providing a convenient foundation model for a variety of data analysis and exploration tasks.
Diffusion Models for High-Resolution Solar Forecasts
Feb 01, 2023



Abstract:Forecasting future weather and climate is inherently difficult. Machine learning offers new approaches to increase the accuracy and computational efficiency of forecasts, but current methods are unable to accurately model uncertainty in high-dimensional predictions. Score-based diffusion models offer a new approach to modeling probability distributions over many dependent variables, and in this work, we demonstrate how they provide probabilistic forecasts of weather and climate variables at unprecedented resolution, speed, and accuracy. We apply the technique to day-ahead solar irradiance forecasts by generating many samples from a diffusion model trained to super-resolve coarse-resolution numerical weather predictions to high-resolution weather satellite observations.
Quantitative Imaging Principles Improves Medical Image Learning
Jun 14, 2022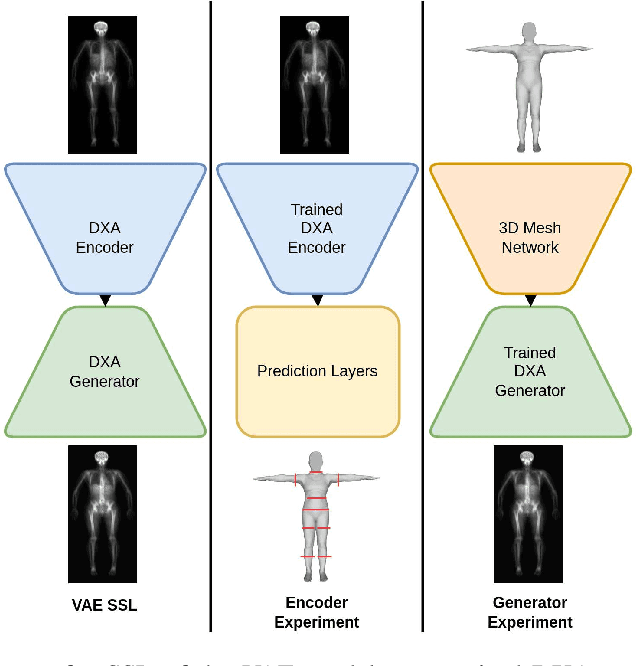
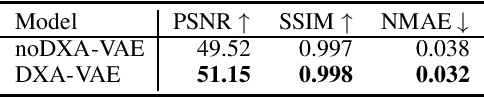
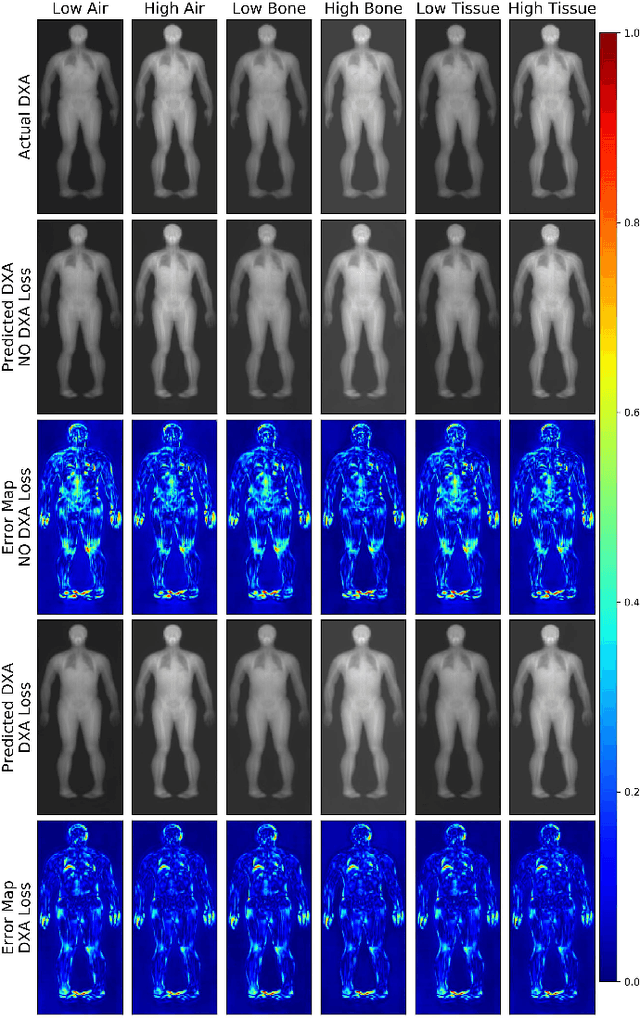
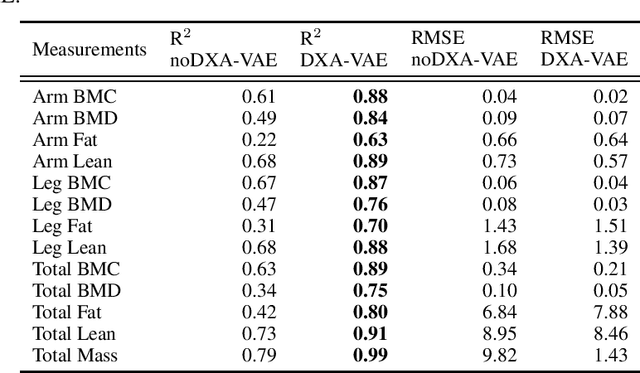
Abstract:Fundamental differences between natural and medical images have recently favored the use of self-supervised learning (SSL) over ImageNet transfer learning for medical image applications. Differences between image types are primarily due to the imaging modality and medical images utilize a wide range of physics based techniques while natural images are captured using only visible light. While many have demonstrated that SSL on medical images has resulted in better downstream task performance, our work suggests that more performance can be gained. The scientific principles which are used to acquire medical images are not often considered when constructing learning problems. For this reason, we propose incorporating quantitative imaging principles during generative SSL to improve image quality and quantitative biological accuracy. We show that this training schema results in better starting states for downstream supervised training on limited data. Our model also generates images that validate on clinical quantitative analysis software.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge