Wenhui Xi
Autism Spectrum Disorder Classification in Children based on Structural MRI Features Extracted using Contrastive Variational Autoencoder
Jul 03, 2023
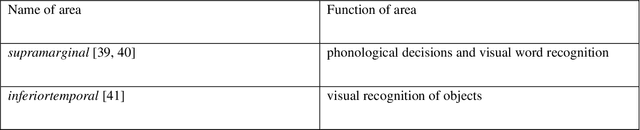


Abstract:Autism spectrum disorder (ASD) is a highly disabling mental disease that brings significant impairments of social interaction ability to the patients, making early screening and intervention of ASD critical. With the development of the machine learning and neuroimaging technology, extensive research has been conducted on machine classification of ASD based on structural MRI (s-MRI). However, most studies involve with datasets where participants' age are above 5. Few studies conduct machine classification of ASD for participants below 5-year-old, but, with mediocre predictive accuracy. In this paper, we push the boundary of predictive accuracy (above 0.97) of machine classification of ASD in children (age range: 0.92-4.83 years), based on s-MRI features extracted using contrastive variational autoencoder (CVAE). 78 s-MRI, collected from Shenzhen Children's Hospital, are used for training CVAE, which consists of both ASD-specific feature channel and common shared feature channel. The ASD participants represented by ASD-specific features can be easily discriminated from TC participants represented by the common shared features, leading to high classification accuracy. In case of degraded predictive accuracy when data size is extremely small, a transfer learning strategy is proposed here as a potential solution. Finally, we conduct neuroanatomical interpretation based on the correlation between s-MRI features extracted from CVAE and surface area of different cortical regions, which discloses potential biomarkers that could help target treatments of ASD in the future.
Identification of Autism spectrum disorder based on a novel feature selection method and Variational Autoencoder
Apr 07, 2022
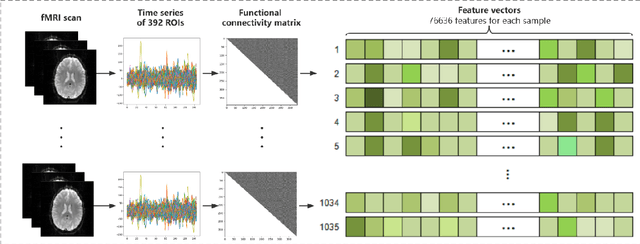
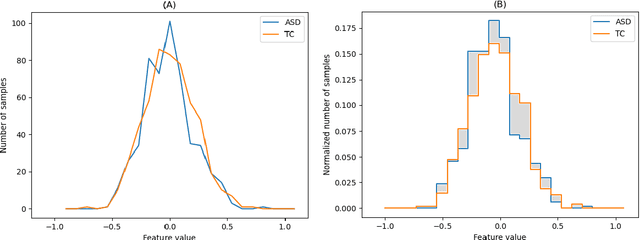
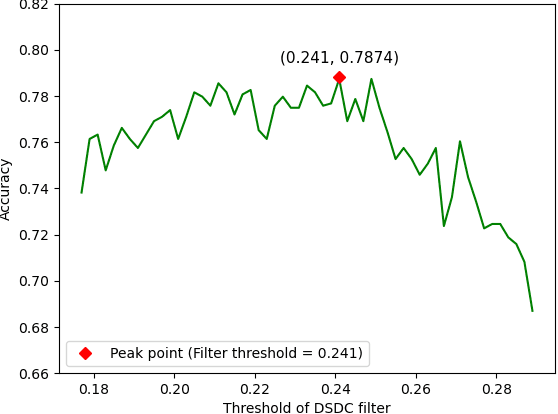
Abstract:The development of noninvasive brain imaging such as resting-state functional magnetic resonance imaging (rs-fMRI) and its combination with AI algorithm provides a promising solution for the early diagnosis of Autism spectrum disorder (ASD). However, the performance of the current ASD classification based on rs-fMRI still needs to be improved. This paper introduces a classification framework to aid ASD diagnosis based on rs-fMRI. In the framework, we proposed a novel filter feature selection method based on the difference between step distribution curves (DSDC) to select remarkable functional connectivities (FCs) and utilized a multilayer perceptron (MLP) which was pretrained by a simplified Variational Autoencoder (VAE) for classification. We also designed a pipeline consisting of a normalization procedure and a modified hyperbolic tangent (tanh) activation function to replace the original tanh function, further improving the model accuracy. Our model was evaluated by 10 times 10-fold cross-validation and achieved an average accuracy of 78.12%, outperforming the state-of-the-art methods reported on the same dataset. Given the importance of sensitivity and specificity in disease diagnosis, two constraints were designed in our model which can improve the model's sensitivity and specificity by up to 9.32% and 10.21%, respectively. The added constraints allow our model to handle different application scenarios and can be used broadly.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge