Varadharajan Jayakumar
Uncertainty aware and explainable diagnosis of retinal disease
Jan 26, 2021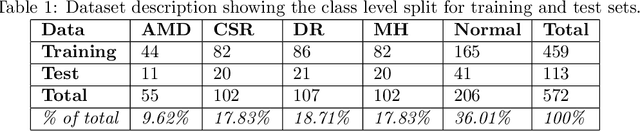
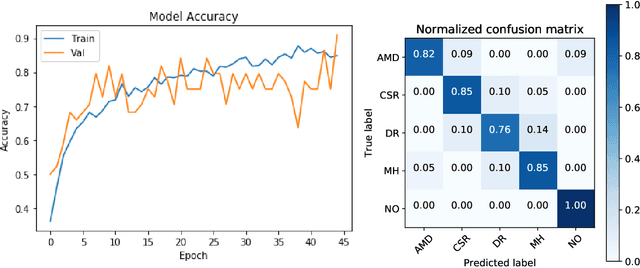
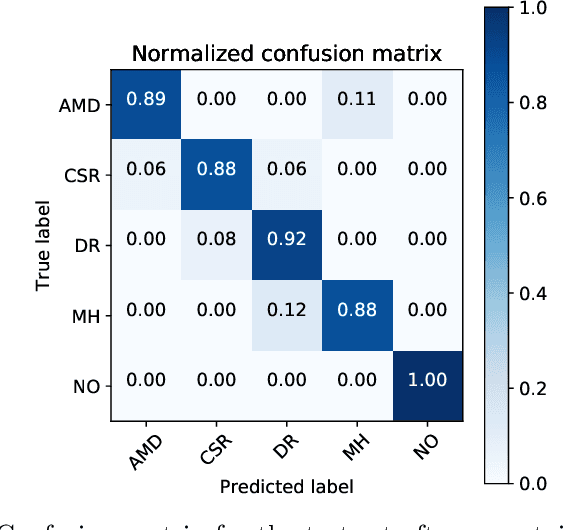
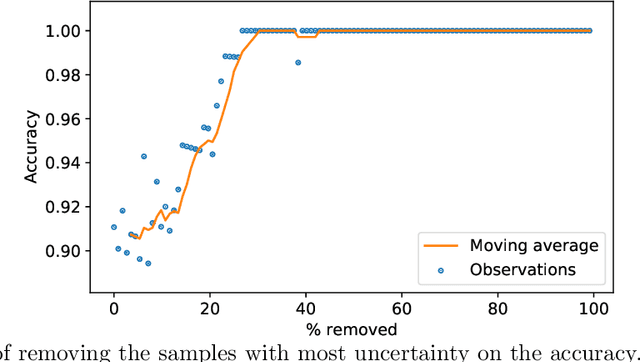
Abstract:Deep learning methods for ophthalmic diagnosis have shown considerable success in tasks like segmentation and classification. However, their widespread application is limited due to the models being opaque and vulnerable to making a wrong decision in complicated cases. Explainability methods show the features that a system used to make prediction while uncertainty awareness is the ability of a system to highlight when it is not sure about the decision. This is one of the first studies using uncertainty and explanations for informed clinical decision making. We perform uncertainty analysis of a deep learning model for diagnosis of four retinal diseases - age-related macular degeneration (AMD), central serous retinopathy (CSR), diabetic retinopathy (DR), and macular hole (MH) using images from a publicly available (OCTID) dataset. Monte Carlo (MC) dropout is used at the test time to generate a distribution of parameters and the predictions approximate the predictive posterior of a Bayesian model. A threshold is computed using the distribution and uncertain cases can be referred to the ophthalmologist thus avoiding an erroneous diagnosis. The features learned by the model are visualized using a proven attribution method from a previous study. The effects of uncertainty on model performance and the relationship between uncertainty and explainability are discussed in terms of clinical significance. The uncertainty information along with the heatmaps make the system more trustworthy for use in clinical settings.
Quantitative and Qualitative Evaluation of Explainable Deep Learning Methods for Ophthalmic Diagnosis
Sep 26, 2020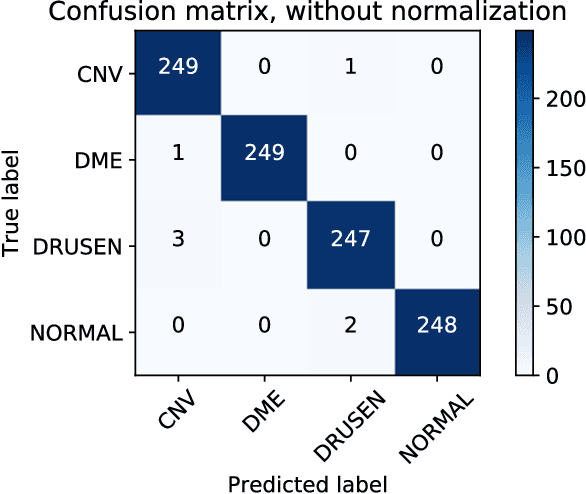
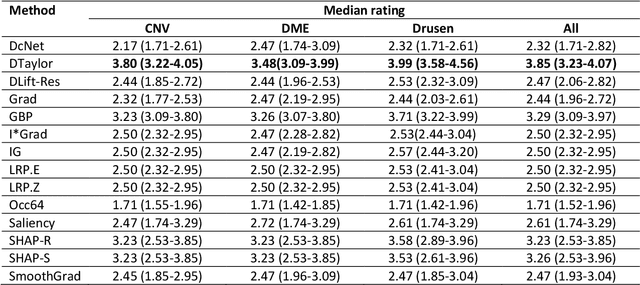
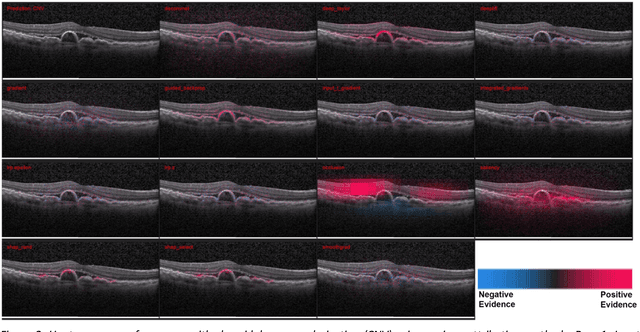
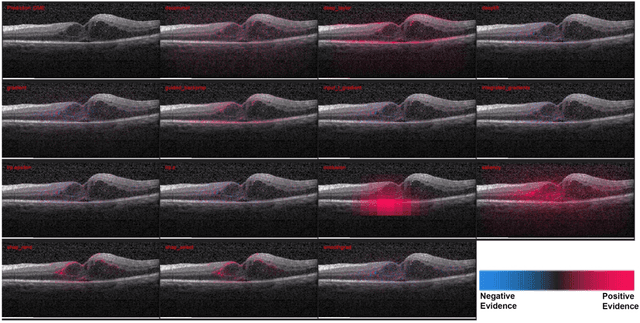
Abstract:Background: The lack of explanations for the decisions made by algorithms such as deep learning has hampered their acceptance by the clinical community despite highly accurate results on multiple problems. Recently, attribution methods have emerged for explaining deep learning models, and they have been tested on medical imaging problems. The performance of attribution methods is compared on standard machine learning datasets and not on medical images. In this study, we perform a comparative analysis to determine the most suitable explainability method for retinal OCT diagnosis. Methods: A commonly used deep learning model known as Inception v3 was trained to diagnose 3 retinal diseases - choroidal neovascularization (CNV), diabetic macular edema (DME), and drusen. The explanations from 13 different attribution methods were rated by a panel of 14 clinicians for clinical significance. Feedback was obtained from the clinicians regarding the current and future scope of such methods. Results: An attribution method based on a Taylor series expansion, called Deep Taylor was rated the highest by clinicians with a median rating of 3.85/5. It was followed by two other attribution methods, Guided backpropagation and SHAP (SHapley Additive exPlanations). Conclusion: Explanations of deep learning models can make them more transparent for clinical diagnosis. This study compared different explanations methods in the context of retinal OCT diagnosis and found that the best performing method may not be the one considered best for other deep learning tasks. Overall, there was a high degree of acceptance from the clinicians surveyed in the study. Keywords: explainable AI, deep learning, machine learning, image processing, Optical coherence tomography, retina, Diabetic macular edema, Choroidal Neovascularization, Drusen
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge