Valentina Bordin
Biomarker Investigation using Multiple Brain Measures from MRI through XAI in Alzheimer's Disease Classification
May 03, 2023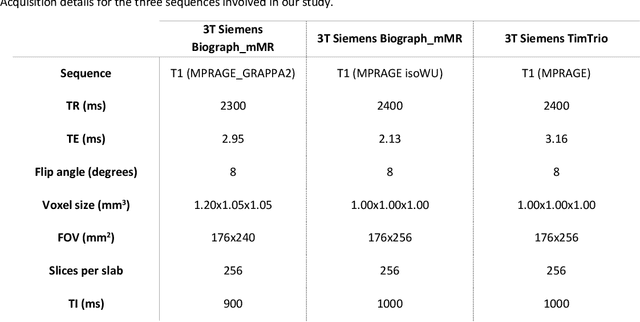
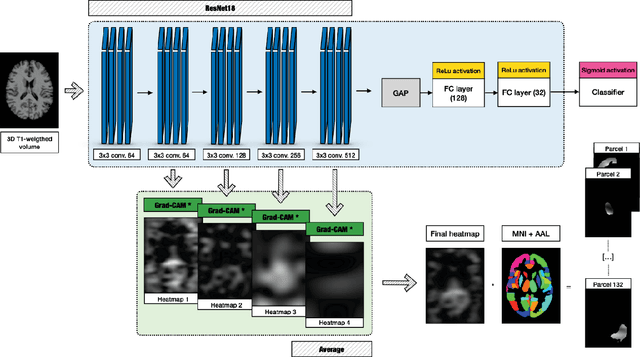
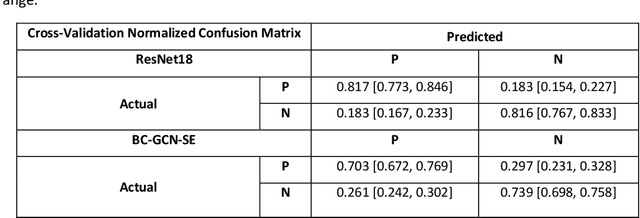
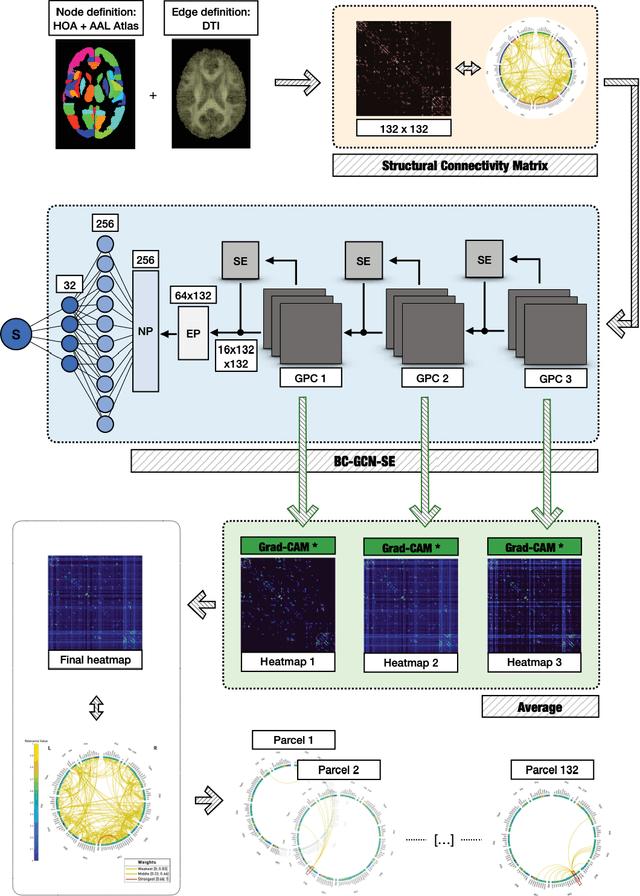
Abstract:Alzheimer's Disease (AD) is the world leading cause of dementia, a progressively impairing condition leading to high hospitalization rates and mortality. To optimize the diagnostic process, numerous efforts have been directed towards the development of deep learning approaches (DL) for the automatic AD classification. However, their typical black box outline has led to low trust and scarce usage within clinical frameworks. In this work, we propose two state-of-the art DL models, trained respectively on structural MRI (ResNet18) and brain connectivity matrixes (BC-GCN-SE) derived from diffusion data. The models were initially evaluated in terms of classification accuracy. Then, results were analyzed using an Explainable Artificial Intelligence (XAI) approach (Grad-CAM) to measure the level of interpretability of both models. The XAI assessment was conducted across 132 brain parcels, extracted from a combination of the Harvard-Oxford and AAL brain atlases, and compared to well-known pathological regions to measure adherence to domain knowledge. Results highlighted acceptable classification performance as compared to the existing literature (ResNet18: TPRmedian = 0.817, TNRmedian = 0.816; BC-GCN-SE: TPRmedian = 0.703, TNRmedian = 0.738). As evaluated through a statistical test (p < 0.05) and ranking of the most relevant parcels (first 15%), Grad-CAM revealed the involvement of target brain areas for both the ResNet18 and BC-GCN-SE models: the medial temporal lobe and the default mode network. The obtained interpretabilities were not without limitations. Nevertheless, results suggested that combining different imaging modalities may result in increased classification performance and model reliability. This could potentially boost the confidence laid in DL models and favor their wide applicability as aid diagnostic tools.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge