Tengjun Liu
Decoding finger velocity from cortical spike trains with recurrent spiking neural networks
Sep 03, 2024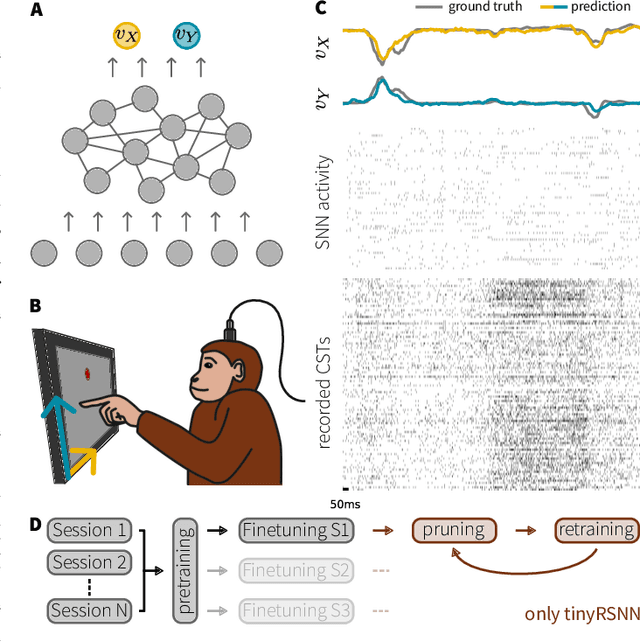
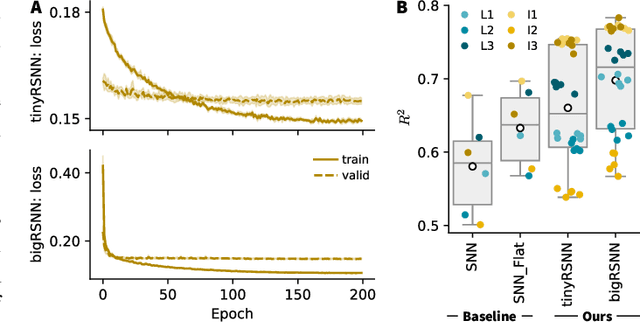
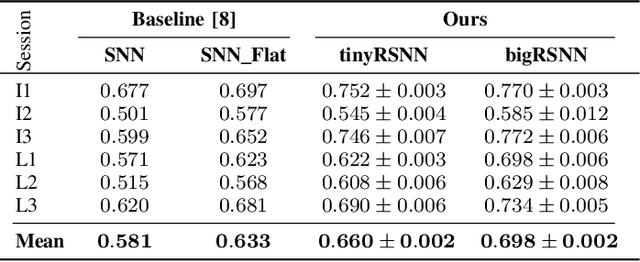
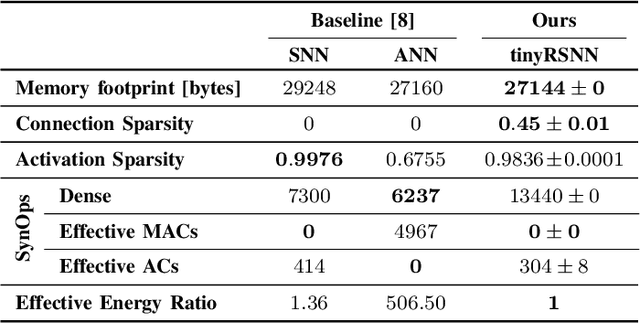
Abstract:Invasive cortical brain-machine interfaces (BMIs) can significantly improve the life quality of motor-impaired patients. Nonetheless, externally mounted pedestals pose an infection risk, which calls for fully implanted systems. Such systems, however, must meet strict latency and energy constraints while providing reliable decoding performance. While recurrent spiking neural networks (RSNNs) are ideally suited for ultra-low-power, low-latency processing on neuromorphic hardware, it is unclear whether they meet the above requirements. To address this question, we trained RSNNs to decode finger velocity from cortical spike trains (CSTs) of two macaque monkeys. First, we found that a large RSNN model outperformed existing feedforward spiking neural networks (SNNs) and artificial neural networks (ANNs) in terms of their decoding accuracy. We next developed a tiny RSNN with a smaller memory footprint, low firing rates, and sparse connectivity. Despite its reduced computational requirements, the resulting model performed substantially better than existing SNN and ANN decoders. Our results thus demonstrate that RSNNs offer competitive CST decoding performance under tight resource constraints and are promising candidates for fully implanted ultra-low-power BMIs with the potential to revolutionize patient care.
Emergent Bio-Functional Similarities in a Cortical-Spike-Train-Decoding Spiking Neural Network Facilitate Predictions of Neural Computation
Mar 14, 2023



Abstract:Despite its better bio-plausibility, goal-driven spiking neural network (SNN) has not achieved applicable performance for classifying biological spike trains, and showed little bio-functional similarities compared to traditional artificial neural networks. In this study, we proposed the motorSRNN, a recurrent SNN topologically inspired by the neural motor circuit of primates. By employing the motorSRNN in decoding spike trains from the primary motor cortex of monkeys, we achieved a good balance between classification accuracy and energy consumption. The motorSRNN communicated with the input by capturing and cultivating more cosine-tuning, an essential property of neurons in the motor cortex, and maintained its stability during training. Such training-induced cultivation and persistency of cosine-tuning was also observed in our monkeys. Moreover, the motorSRNN produced additional bio-functional similarities at the single-neuron, population, and circuit levels, demonstrating biological authenticity. Thereby, ablation studies on motorSRNN have suggested long-term stable feedback synapses contribute to the training-induced cultivation in the motor cortex. Besides these novel findings and predictions, we offer a new framework for building authentic models of neural computation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge