Stephen R. Aylward
Fluid registration between lung CT and stationary chest tomosynthesis images
Mar 06, 2022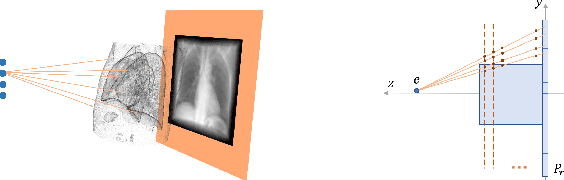
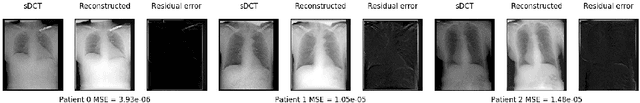
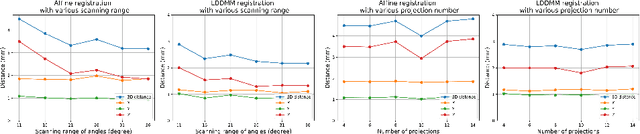
Abstract:Registration is widely used in image-guided therapy and image-guided surgery to estimate spatial correspondences between organs of interest between planning and treatment images. However, while high-quality computed tomography (CT) images are often available at planning time, limited angle acquisitions are frequently used during treatment because of radiation concerns or imaging time constraints. This requires algorithms to register CT images based on limited angle acquisitions. We, therefore, formulate a 3D/2D registration approach which infers a 3D deformation based on measured projections and digitally reconstructed radiographs of the CT. Most 3D/2D registration approaches use simple transformation models or require complex mathematical derivations to formulate the underlying optimization problem. Instead, our approach entirely relies on differentiable operations which can be combined with modern computational toolboxes supporting automatic differentiation. This then allows for rapid prototyping, integration with deep neural networks, and to support a variety of transformation models including fluid flow models. We demonstrate our approach for the registration between CT and stationary chest tomosynthesis (sDCT) images and show how it naturally leads to an iterative image reconstruction approach.
Discovering Hidden Physics Behind Transport Dynamics
Nov 24, 2020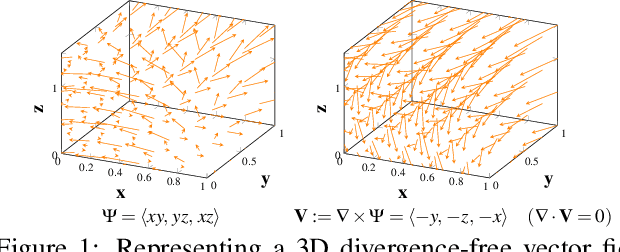
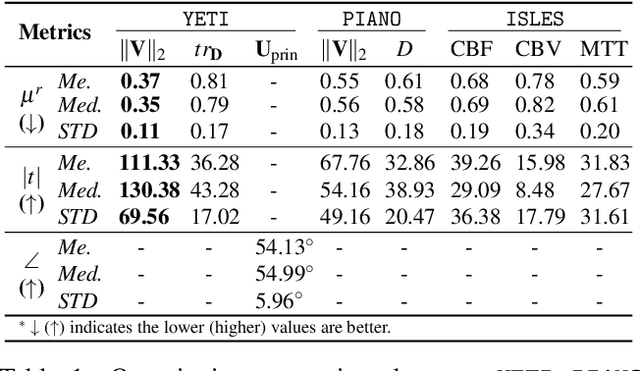
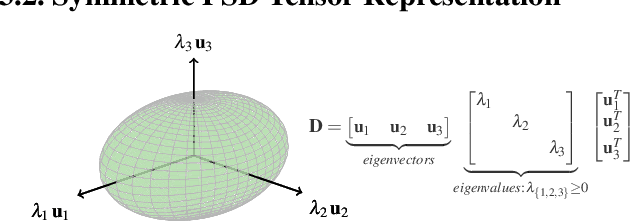
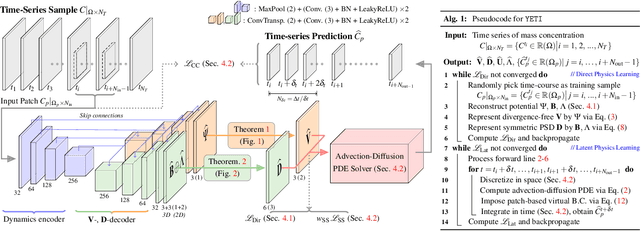
Abstract:Transport processes are ubiquitous. They are, for example, at the heart of optical flow approaches; or of perfusion imaging, where blood transport is assessed, most commonly by injecting a tracer. An advection-diffusion equation is widely used to describe these transport phenomena. Our goal is estimating the underlying physics of advection-diffusion equations, expressed as velocity and diffusion tensor fields. We propose a learning framework (YETI) building on an auto-encoder structure between 2D and 3D image time-series, which incorporates the advection-diffusion model. To help with identifiability, we develop an advection-diffusion simulator which allows pre-training of our model by supervised learning using the velocity and diffusion tensor fields. Instead of directly learning these velocity and diffusion tensor fields, we introduce representations that assure incompressible flow and symmetric positive semi-definite diffusion fields and demonstrate the additional benefits of these representations on improving estimation accuracy. We further use transfer learning to apply YETI on a public brain magnetic resonance (MR) perfusion dataset of stroke patients and show its ability to successfully distinguish stroke lesions from normal brain regions via the estimated velocity and diffusion tensor fields.
Perfusion Imaging: A Data Assimilation Approach
Sep 06, 2020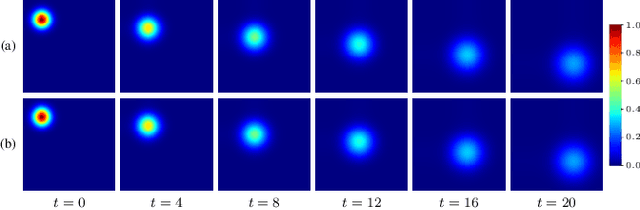
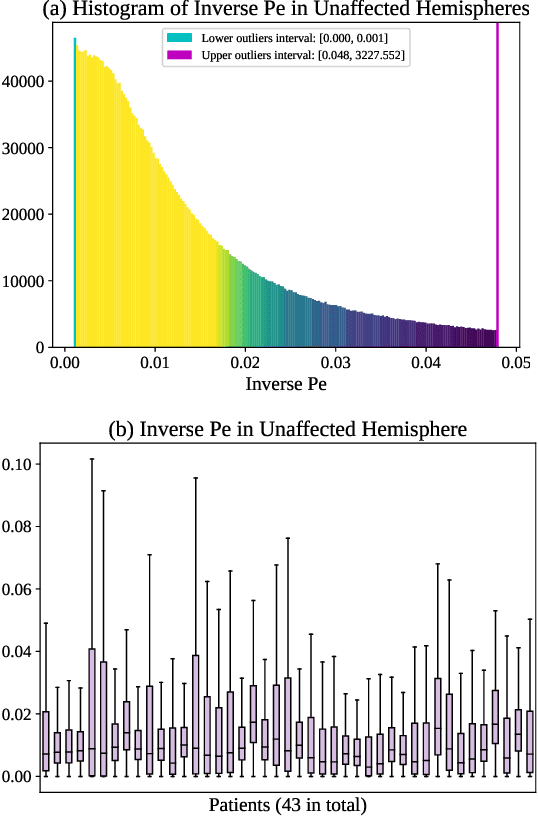
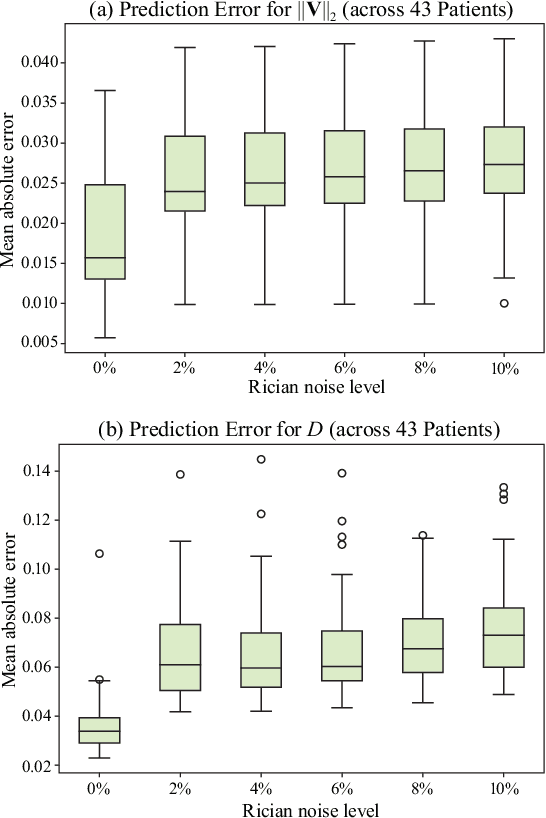
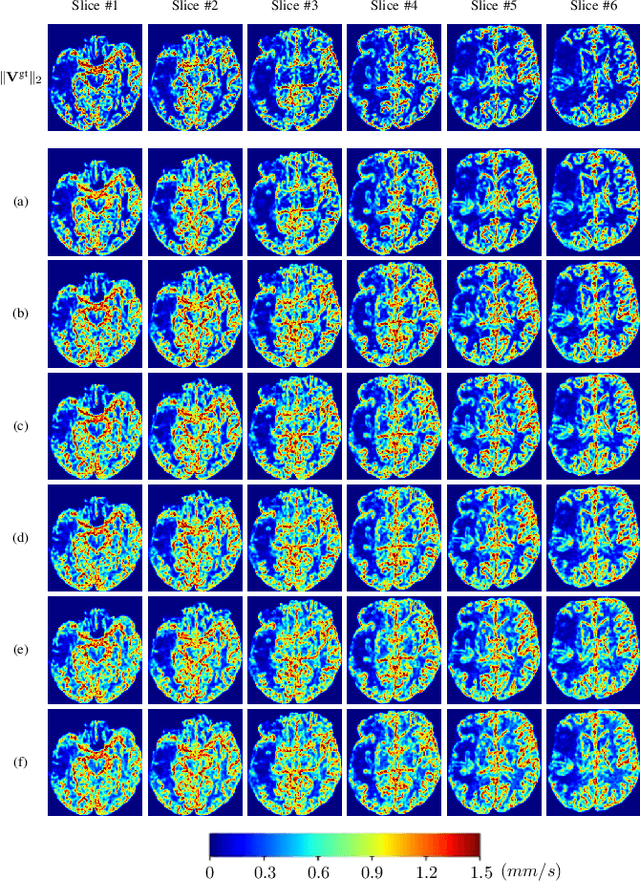
Abstract:Perfusion imaging (PI) is clinically used to assess strokes and brain tumors. Commonly used PI approaches based on magnetic resonance imaging (MRI) or computed tomography (CT) measure the effect of a contrast agent moving through blood vessels and into tissue. Contrast-agent free approaches, for example, based on intravoxel incoherent motion, also exist, but are so far not routinely used clinically. These methods rely on estimating on the arterial input function (AIF) to approximately model tissue perfusion, neglecting spatial dependencies, and reliably estimating the AIF is also non-trivial, leading to difficulties with standardizing perfusion measures. In this work we therefore propose a data-assimilation approach (PIANO) which estimates the velocity and diffusion fields of an advection-diffusion model that best explains the contrast dynamics. PIANO accounts for spatial dependencies and neither requires estimating the AIF nor relies on a particular contrast agent bolus shape. Specifically, we propose a convenient parameterization of the estimation problem, a numerical estimation approach, and extensively evaluate PIANO. We demonstrate that PIANO can successfully resolve velocity and diffusion field ambiguities and results in sensitive measures for the assessment of stroke, comparing favorably to conventional measures of perfusion.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge