Stephanie Leung
Direct Automated Quantitative Measurement of Spine via Cascade Amplifier Regression Network
Jun 14, 2018


Abstract:Automated quantitative measurement of the spine (i.e., multiple indices estimation of heights, widths, areas, and so on for the vertebral body and disc) is of the utmost importance in clinical spinal disease diagnoses, such as osteoporosis, intervertebral disc degeneration, and lumbar disc herniation, yet still an unprecedented challenge due to the variety of spine structure and the high dimensionality of indices to be estimated. In this paper, we propose a novel cascade amplifier regression network (CARN), which includes the CARN architecture and local shape-constrained manifold regularization (LSCMR) loss function, to achieve accurate direct automated multiple indices estimation. The CARN architecture is composed of a cascade amplifier network (CAN) for expressive feature embedding and a linear regression model for multiple indices estimation. The CAN consists of cascade amplifier units (AUs), which are used for selective feature reuse by stimulating effective feature and suppressing redundant feature during propagating feature map between adjacent layers, thus an expressive feature embedding is obtained. During training, the LSCMR is utilized to alleviate overfitting and generate realistic estimation by learning the multiple indices distribution. Experiments on MR images of 195 subjects show that the proposed CARN achieves impressive performance with mean absolute errors of 1.2496 mm, 1.2887 mm, and 1.2692 mm for estimation of 15 heights of discs, 15 heights of vertebral bodies, and total indices respectively. The proposed method has great potential in clinical spinal disease diagnoses.
Cardiac Motion Scoring with Segment- and Subject-level Non-Local Modeling
Jun 14, 2018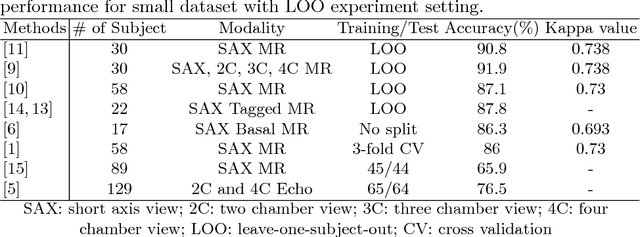
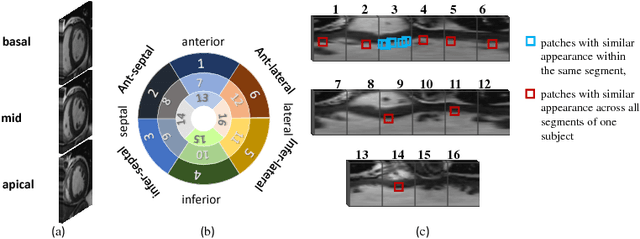
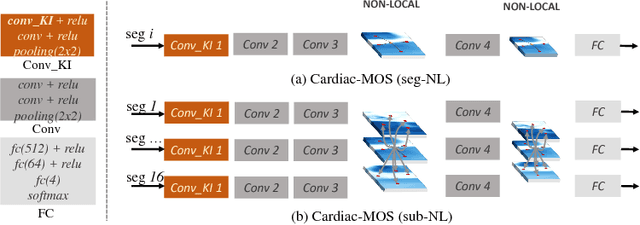

Abstract:Motion scoring of cardiac myocardium is of paramount importance for early detection and diagnosis of various cardiac disease. It aims at identifying regional wall motions into one of the four types including normal, hypokinetic, akinetic, and dyskinetic, and is extremely challenging due to the complex myocardium deformation and subtle inter-class difference of motion patterns. All existing work on automated motion analysis are focused on binary abnormality detection to avoid the much more demanding motion scoring, which is urgently required in real clinical practice yet has never been investigated before. In this work, we propose Cardiac-MOS, the first powerful method for cardiac motion scoring from cardiac MR sequences based on deep convolution neural network. Due to the locality of convolution, the relationship between distant non-local responses of the feature map cannot be explored, which is closely related to motion difference between segments. In Cardiac-MOS, such non-local relationship is modeled with non-local neural network within each segment and across all segments of one subject, i.e., segment- and subject-level non-local modeling, and lead to obvious performance improvement. Besides, Cardiac-MOS can effectively extract motion information from MR sequences of various lengths by interpolating the convolution kernel along the temporal dimension, therefore can be applied to MR sequences of multiple sources. Experiments on 1440 myocardium segments of 90 subjects from short axis MR sequences of multiple lengths prove that Cardiac-MOS achieves reliable performance, with correlation of 0.926 for motion score index estimation and accuracy of 77.4\% for motion scoring. Cardiac-MOS also outperforms all existing work for binary abnormality detection. As the first automatic motion scoring solution, Cardiac-MOS demonstrates great potential in future clinical application.
Direct Estimation of Regional Wall Thicknesses via Residual Recurrent Neural Network
May 26, 2017



Abstract:Accurate estimation of regional wall thicknesses (RWT) of left ventricular (LV) myocardium from cardiac MR sequences is of significant importance for identification and diagnosis of cardiac disease. Existing RWT estimation still relies on segmentation of LV myocardium, which requires strong prior information and user interaction. No work has been devoted into direct estimation of RWT from cardiac MR images due to the diverse shapes and structures for various subjects and cardiac diseases, as well as the complex regional deformation of LV myocardium during the systole and diastole phases of the cardiac cycle. In this paper, we present a newly proposed Residual Recurrent Neural Network (ResRNN) that fully leverages the spatial and temporal dynamics of LV myocardium to achieve accurate frame-wise RWT estimation. Our ResRNN comprises two paths: 1) a feed forward convolution neural network (CNN) for effective and robust CNN embedding learning of various cardiac images and preliminary estimation of RWT from each frame itself independently, and 2) a recurrent neural network (RNN) for further improving the estimation by modeling spatial and temporal dynamics of LV myocardium. For the RNN path, we design for cardiac sequences a Circle-RNN to eliminate the effect of null hidden input for the first time-step. Our ResRNN is capable of obtaining accurate estimation of cardiac RWT with Mean Absolute Error of 1.44mm (less than 1-pixel error) when validated on cardiac MR sequences of 145 subjects, evidencing its great potential in clinical cardiac function assessment.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge