Skylar Chan
Quantum State Fidelity for Functional Neural Network Construction
Aug 23, 2025Abstract:Neuroscientists face challenges in analyzing high-dimensional neural recording data of dense functional networks. Without ground-truth reference data, finding the best algorithm for recovering neurologically relevant networks remains an open question. We implemented hybrid quantum algorithms to construct functional networks and compared them with the results of documented classical techniques. We demonstrated that our quantum state fidelity can provide a competitive alternative to classical metrics by revealing distinct functional networks. Our results suggest that quantum computing offers a viable and potentially advantageous alternative for data-driven modeling in neuroscience, underscoring its broader applicability in high-dimensional graph inference and complex system analysis.
Expanding the Horizon: Enabling Hybrid Quantum Transfer Learning for Long-Tailed Chest X-Ray Classification
Apr 30, 2024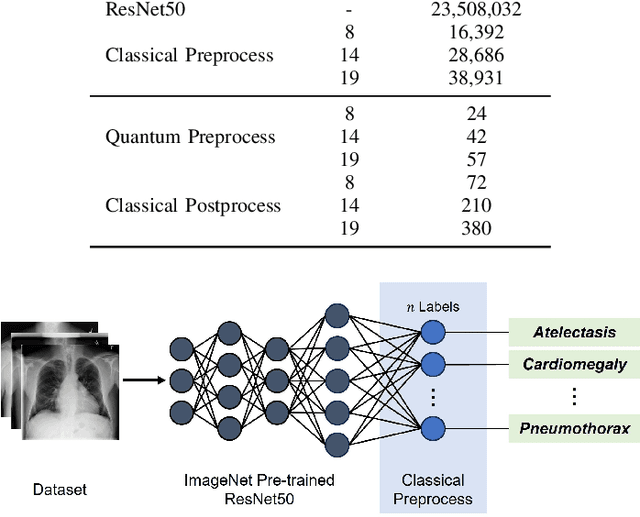
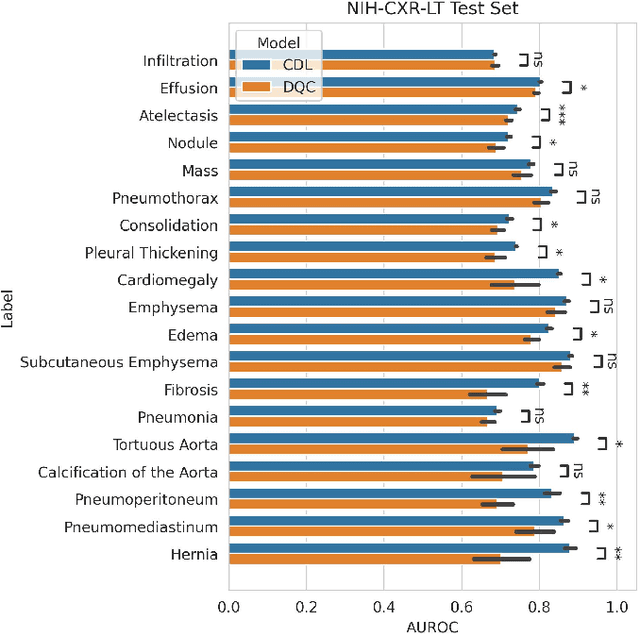
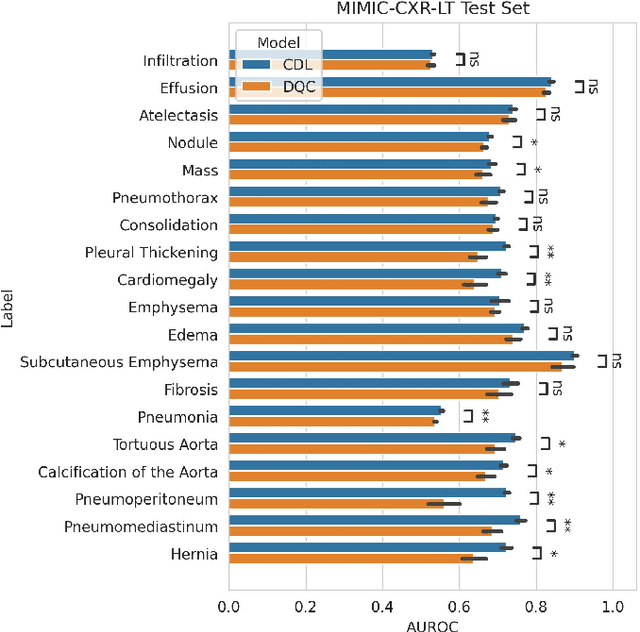
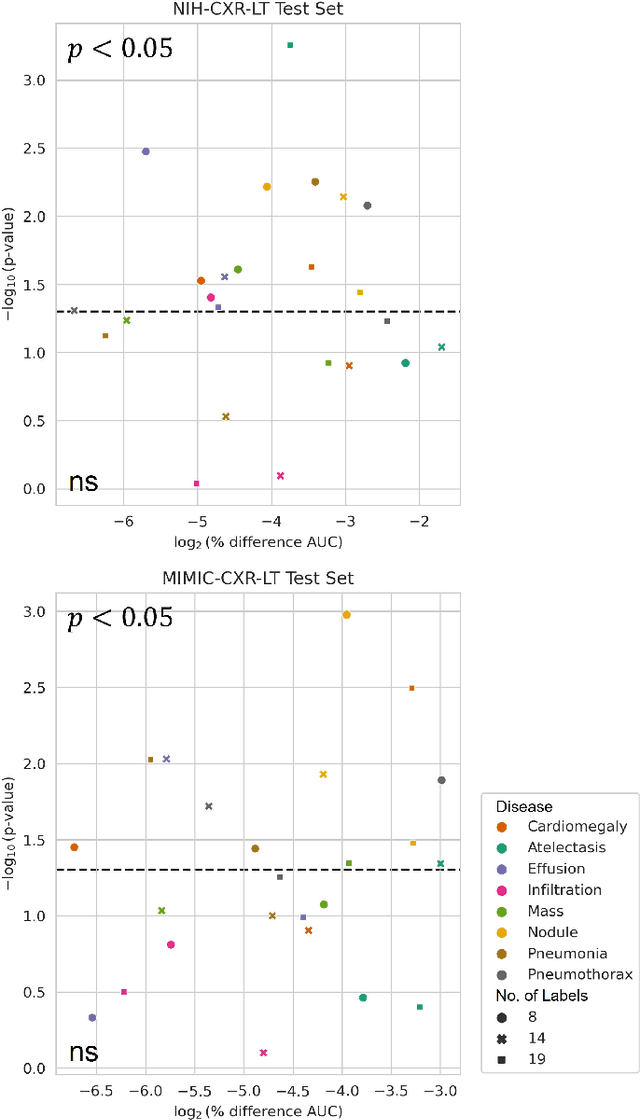
Abstract:Quantum machine learning (QML) has the potential for improving the multi-label classification of rare, albeit critical, diseases in large-scale chest x-ray (CXR) datasets due to theoretical quantum advantages over classical machine learning (CML) in sample efficiency and generalizability. While prior literature has explored QML with CXRs, it has focused on binary classification tasks with small datasets due to limited access to quantum hardware and computationally expensive simulations. To that end, we implemented a Jax-based framework that enables the simulation of medium-sized qubit architectures with significant improvements in wall-clock time over current software offerings. We evaluated the performance of our Jax-based framework in terms of efficiency and performance for hybrid quantum transfer learning for long-tailed classification across 8, 14, and 19 disease labels using large-scale CXR datasets. The Jax-based framework resulted in up to a 58% and 95% speed-up compared to PyTorch and TensorFlow implementations, respectively. However, compared to CML, QML demonstrated slower convergence and an average AUROC of 0.70, 0.73, and 0.74 for the classification of 8, 14, and 19 CXR disease labels. In comparison, the CML models had an average AUROC of 0.77, 0.78, and 0.80 respectively. In conclusion, our work presents an accessible implementation of hybrid quantum transfer learning for long-tailed CXR classification with a computationally efficient Jax-based framework.
Hidden in Plain Sight: Undetectable Adversarial Bias Attacks on Vulnerable Patient Populations
Feb 08, 2024Abstract:The proliferation of artificial intelligence (AI) in radiology has shed light on the risk of deep learning (DL) models exacerbating clinical biases towards vulnerable patient populations. While prior literature has focused on quantifying biases exhibited by trained DL models, demographically targeted adversarial bias attacks on DL models and its implication in the clinical environment remains an underexplored field of research in medical imaging. In this work, we demonstrate that demographically targeted label poisoning attacks can introduce adversarial underdiagnosis bias in DL models and degrade performance on underrepresented groups without impacting overall model performance. Moreover, our results across multiple performance metrics and demographic groups like sex, age, and their intersectional subgroups indicate that a group's vulnerability to undetectable adversarial bias attacks is directly correlated with its representation in the model's training data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge