Simon J. Ward
Sensor Response-Time Reduction using Long-Short Term Memory Network Forecasting
Apr 26, 2024Abstract:The response time of a biosensor is a crucial metric in safety-critical applications such as medical diagnostics where an earlier diagnosis can markedly improve patient outcomes. However, the speed at which a biosensor reaches a final equilibrium state can be limited by poor mass transport and long molecular diffusion times that increase the time it takes target molecules to reach the active sensing region of a biosensor. While optimization of system and sensor design can promote molecules reaching the sensing element faster, a simpler and complementary approach for response time reduction that is widely applicable across all sensor platforms is to use time-series forecasting to predict the ultimate steady-state sensor response. In this work, we show that ensembles of long short-term memory (LSTM) networks can accurately predict equilibrium biosensor response from a small quantity of initial time-dependent biosensor measurements, allowing for significant reduction in response time by a mean and median factor of improvement of 18.6 and 5.1, respectively. The ensemble of models also provides simultaneous estimation of uncertainty, which is vital to provide confidence in the predictions and subsequent safety-related decisions that are made. This approach is demonstrated on real-time experimental data collected by exposing porous silicon biosensors to buffered protein solutions using a multi-channel fluidic cell that enables the automated measurement of 100 porous silicon biosensors in parallel. The dramatic improvement in sensor response time achieved using LSTM network ensembles and associated uncertainty quantification opens the door to trustworthy and faster responding biosensors, enabling more rapid medical diagnostics for improved patient outcomes and healthcare access, as well as quicker identification of toxins in food and the environment.
Capture Agent Free Biosensing using Porous Silicon Arrays and Machine Learning
Jan 22, 2022



Abstract:Biosensors are an essential tool for medical diagnostics, environmental monitoring and food safety. Typically, biosensors are designed to detect specific analytes through functionalization with the appropriate capture agents. However, the use of capture agents limits the number of analytes that can be simultaneously detected and reduces the robustness of the biosensor. In this work, we report a versatile, capture agent free biosensor platform based on an array of porous silicon (PSi) thin films, which has the potential to robustly detect a wide variety of analytes based on their physical and chemical properties in the nanoscale porous media. The ability of this system to reproducibly classify, quantify, and discriminate three proteins is demonstrated to concentrations down to at least 0.02g/L (between 300nM and 450nM) by utilizing PSi array elements with a unique combination of pore size and buffer pH, employing linear discriminant analysis for dimensionality reduction, and using support vector machines as a classifier. This approach represents a significant step towards a low cost, simple and robust biosensor platform that is able to detect a vast range of biomolecules.
Signal Processing Techniques to Reduce the Limit of Detection for Thin Film Biosensors
Mar 12, 2021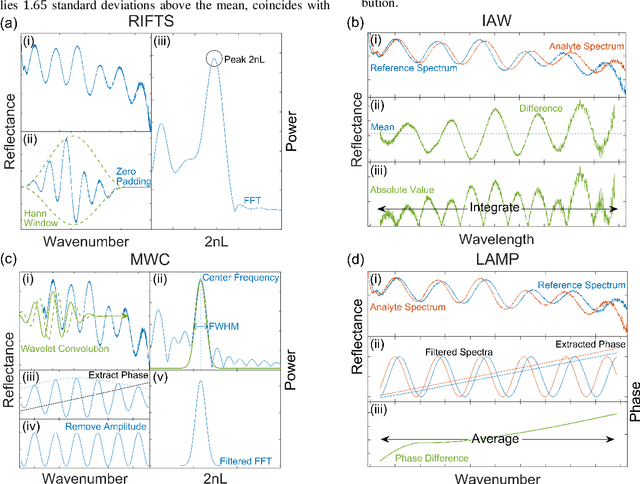
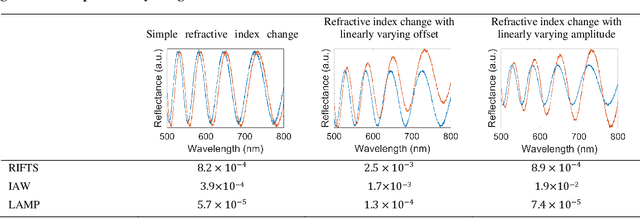


Abstract:The ultimate detection limit of optical biosensors is often limited by various noise sources, including those introduced by the optical measurement setup. While sophisticated modifications to instrumentation may reduce noise, a simpler approach that can benefit all sensor platforms is the application of signal processing to minimize the deleterious effects of noise. In this work, we show that applying complex Morlet wavelet convolution to Fabry-P\'erot interference fringes characteristic of thin film reflectometric biosensors effectively filters out white noise and low frequency reflectance variations. Subsequent calculation of an average difference in phase between the filtered analyte and reference signals enables a significant reduction in the limit of detection (LOD) enabling closer competition with current state-of-the-art techniques. This method is applied on experimental data sets of thin film porous silicon sensors (PSi) in buffered solution and complex media obtained from two different laboratories. The demonstrated improvement in LOD achieved using wavelet convolution and average phase difference paves the way for PSi optical biosensors to operate with clinically relevant detection limits for medical diagnostics, environmental monitoring, and food safety.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge