Sebastian Meller
Embedding digital chronotherapy into medical devices -- A canine case study in controlling status epilepticus through multi-scale rhythmic brain stimulation
Jul 07, 2021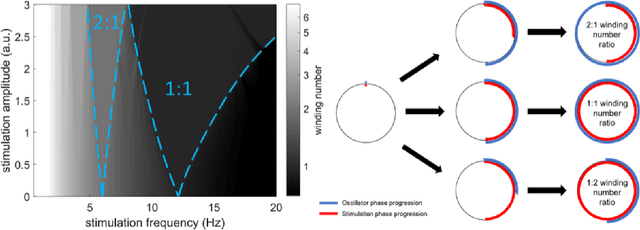
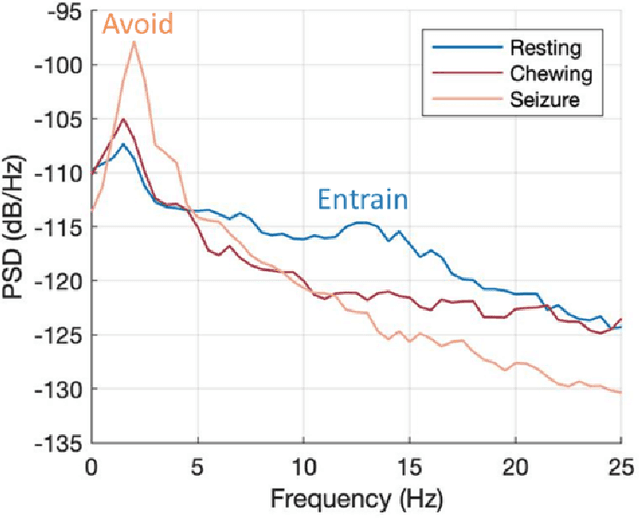
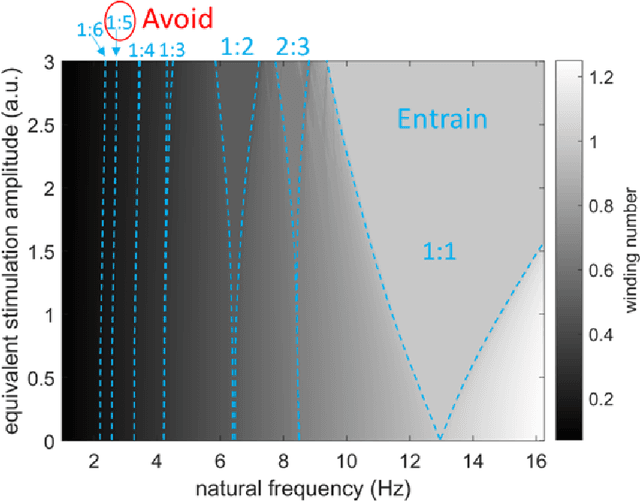

Abstract:Circadian and other physiological rhythms play a key role in both normal homeostasis and disease processes. Such is the case of circadian and infradian seizure patterns observed in epilepsy. However, these rhythms are not fully exploited in the design of active implantable medical devices. In this paper we explore a new implantable stimulator that implements chronotherapy as a feedforward input to supplement both open-loop and closed-loop methods. This integrated algorithm allows for stimulation to be adjusted to the ultradian, circadian, and infradian patterns observed in patients through slowly-varying temporal adjustments of stimulation and algorithm sub-components, while also enabling adaption of stimulation based on immediate physiological needs such as a breakthrough seizure or change of posture. Embedded physiological sensors in the stimulator can be used to refine the baseline stimulation circadian pattern as a "digital zeitgeber". This algorithmic approach is tested on a canine with severe drug-resistant idiopathic generalized epilepsy exhibiting a characteristic diurnal pattern correlated with sleep-wake cycles. Prior to implantation, the canine's cluster seizures evolved to status epilepticus (SE) and required emergency pharmacological intervention. The cranially-mounted system was fully-implanted bilaterally into the centromedian nucleus of the thalamus. Using combinations of time-based modulation, thalamocortical rhythm-specific tuning of frequency parameters, and fast-adaptive modes based on activity, the canine has experienced no further SE events post-implant at the time of writing (7 months), and no significant clusters are observed any longer. The use of digitally-enabled chronotherapy as a feedforward signal to augment adaptive neurostimulators could prove a useful algorithmic method where sensitivity to temporal patterns are characteristics of the disease state.
A New 3D Method to Segment the Lumbar Vertebral Bodies and to Determine Bone Mineral Density and Geometry
May 19, 2017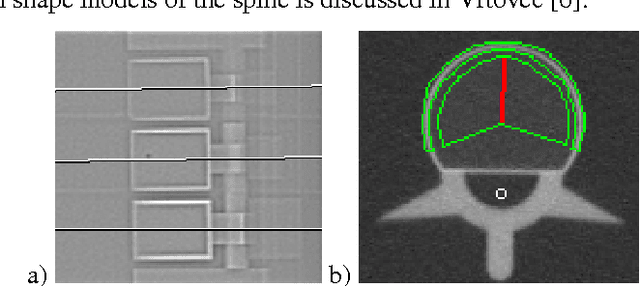
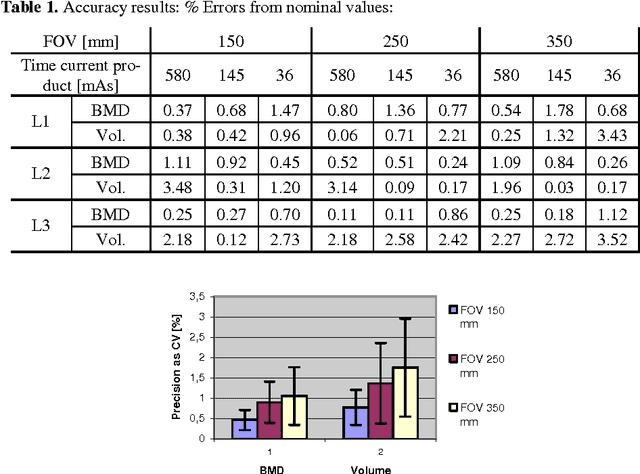
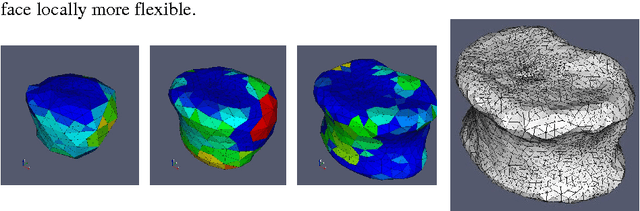

Abstract:In this paper we present a new 3D segmentation approach for the vertebrae of the lower thoracic and the lumbar spine in spiral computed tomography datasets. We implemented a multi-step procedure. Its main components are deformable models, volume growing, and morphological operations. The performance analysis that included an evaluation of accuracy using the European Spine Phantom, and of intra-operator precision using clinical CT datasets from 10 patients highlight the potential for clinical use. The intra-operator precision of the segmentation procedure was better than 1% for Bone Mineral Density (BMD) and better than 1.8% for volume. The long-term goal of this work is to enable better fracture prediction and improved patient monitoring in the field of osteoporosis. A true 3D segmentation also enables an accurate measurement of geometrical parameters that can augment the classical measurement of BMD.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge