Sebastian J. Crutch
for the Alzheimer's Disease Neuroimaging Initiative
DIVE: A spatiotemporal progression model of brain pathology in neurodegenerative disorders
Jan 11, 2019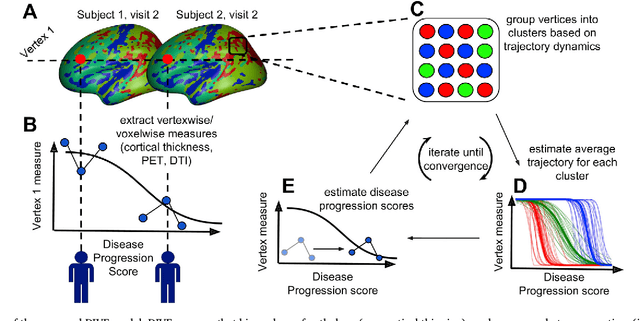
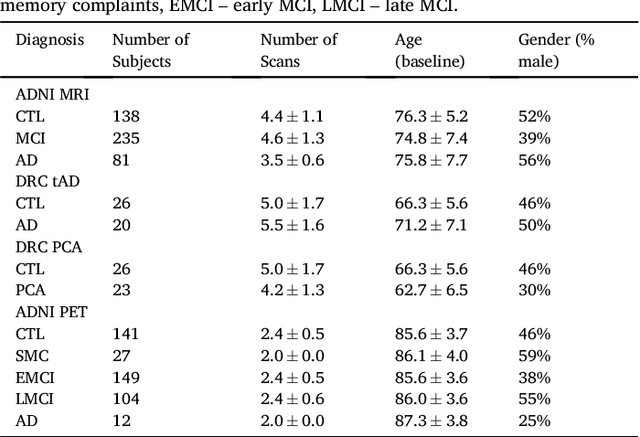
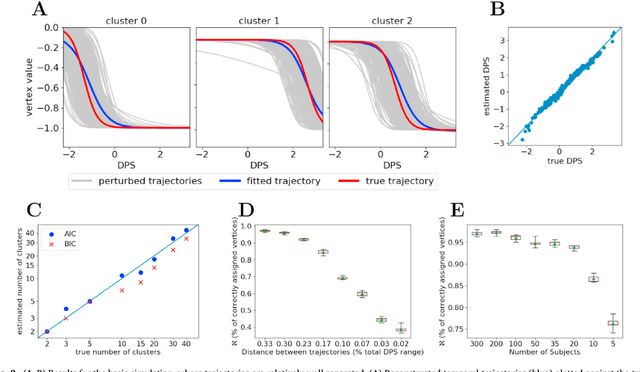
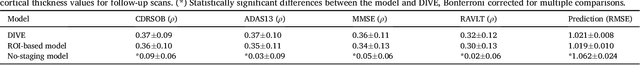
Abstract:Here we present DIVE: Data-driven Inference of Vertexwise Evolution. DIVE is an image-based disease progression model with single-vertex resolution, designed to reconstruct long-term patterns of brain pathology from short-term longitudinal data sets. DIVE clusters vertex-wise biomarker measurements on the cortical surface that have similar temporal dynamics across a patient population, and concurrently estimates an average trajectory of vertex measurements in each cluster. DIVE uniquely outputs a parcellation of the cortex into areas with common progression patterns, leading to a new signature for individual diseases. DIVE further estimates the disease stage and progression speed for every visit of every subject, potentially enhancing stratification for clinical trials or management. On simulated data, DIVE can recover ground truth clusters and their underlying trajectory, provided the average trajectories are sufficiently different between clusters. We demonstrate DIVE on data from two cohorts: the Alzheimer's Disease Neuroimaging Initiative (ADNI) and the Dementia Research Centre (DRC), UK, containing patients with Posterior Cortical Atrophy (PCA) as well as typical Alzheimer's disease (tAD). DIVE finds similar spatial patterns of atrophy for tAD subjects in the two independent datasets (ADNI and DRC), and further reveals distinct patterns of pathology in different diseases (tAD vs PCA) and for distinct types of biomarker data: cortical thickness from Magnetic Resonance Imaging (MRI) vs amyloid load from Positron Emission Tomography (PET). Finally, DIVE can be used to estimate a fine-grained spatial distribution of pathology in the brain using any kind of voxelwise or vertexwise measures including Jacobian compression maps, fractional anisotropy (FA) maps from diffusion imaging or other PET measures. DIVE source code is available online: https://github.com/mrazvan22/dive
Disease Knowledge Transfer across Neurodegenerative Diseases
Jan 11, 2019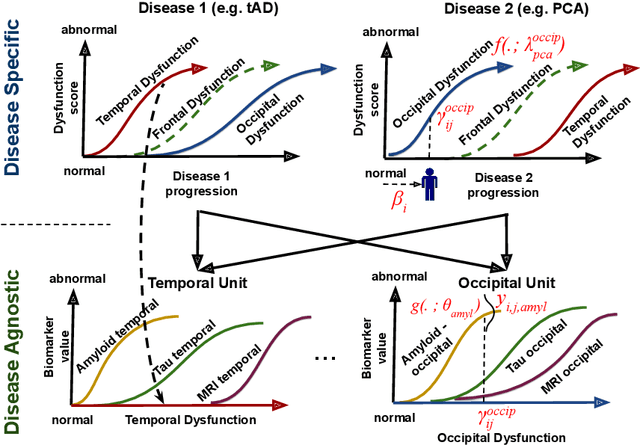
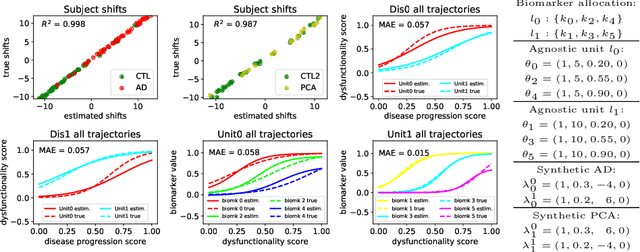
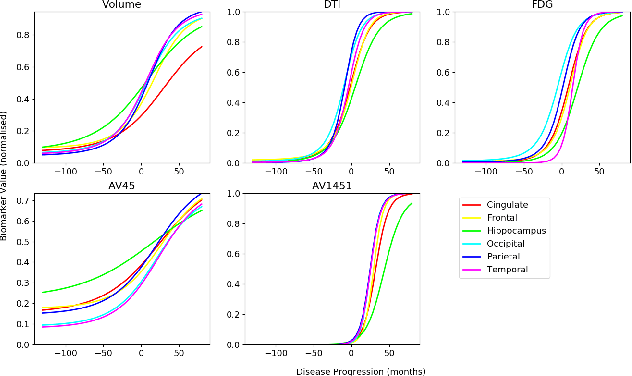
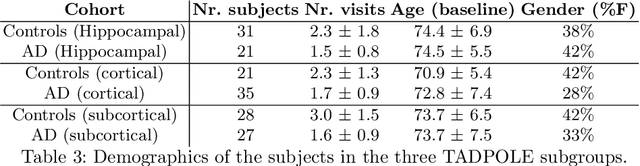
Abstract:We introduce Disease Knowledge Transfer (DKT), a novel technique for transferring biomarker information between related neurodegenerative diseases. DKT infers robust multimodal biomarker trajectories in rare neurodegenerative diseases even when only limited, unimodal data is available, by transferring information from larger multimodal datasets from common neurodegenerative diseases. DKT is a joint-disease generative model of biomarker progressions, which exploits biomarker relationships that are shared across diseases. As opposed to current deep learning approaches, DKT is interpretable, which allows us to understand underlying disease mechanisms. Here we demonstrate DKT on Alzheimer's disease (AD) variants and its ability to predict trajectories for multimodal biomarkers in Posterior Cortical Atrophy (PCA), in lack of such data from PCA subjects. For this we train DKT on a combined dataset containing subjects with two distinct diseases and sizes of data available: 1) a larger, multimodal typical AD (tAD) dataset from the TADPOLE Challenge, and 2) a smaller unimodal Posterior Cortical Atrophy (PCA) dataset from the Dementia Research Centre (DRC) UK, for which only a limited number of Magnetic Resonance Imaging (MRI) scans are available. We first show that DKT estimates plausible multimodal trajectories in PCA that agree with previous literature. We further validate DKT in two situations: (1) on synthetic data, showing that it can accurately estimate the ground truth parameters and (2) on 20 DTI scans from controls and PCA patients, showing that it has favourable predictive performance compared to standard approaches. While we demonstrated DKT on Alzheimer's variants, we note DKT is generalisable to other forms of related neurodegenerative diseases. Source code for DKT is available online: https://github.com/mrazvan22/dkt.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge