Sarah E. Bohndiek
Digital twins enable full-reference quality assessment of photoacoustic image reconstructions
May 30, 2025



Abstract:Quantitative comparison of the quality of photoacoustic image reconstruction algorithms remains a major challenge. No-reference image quality measures are often inadequate, but full-reference measures require access to an ideal reference image. While the ground truth is known in simulations, it is unknown in vivo, or in phantom studies, as the reference depends on both the phantom properties and the imaging system. We tackle this problem by using numerical digital twins of tissue-mimicking phantoms and the imaging system to perform a quantitative calibration to reduce the simulation gap. The contributions of this paper are two-fold: First, we use this digital-twin framework to compare multiple state-of-the-art reconstruction algorithms. Second, among these is a Fourier transform-based reconstruction algorithm for circular detection geometries, which we test on experimental data for the first time. Our results demonstrate the usefulness of digital phantom twins by enabling assessment of the accuracy of the numerical forward model and enabling comparison of image reconstruction schemes with full-reference image quality assessment. We show that the Fourier transform-based algorithm yields results comparable to those of iterative time reversal, but at a lower computational cost. All data and code are publicly available on Zenodo: https://doi.org/10.5281/zenodo.15388429.
Hyperpixels: Pixel Filter Arrays of Multivariate Optical Elements for Optimized Spectral Imaging
Mar 25, 2024



Abstract:We introduce the concept of `hyperpixels' in which each element of a pixel filter array (suitable for CMOS image sensor integration) has a spectral transmission tailored to a target spectral component expected in application-specific scenes. These are analogous to arrays of multivariate optical elements that could be used for sensing specific analytes. Spectral tailoring is achieved by engineering the heights of multiple sub-pixel Fabry-Perot resonators that cover each pixel area. We first present a design approach for hyperpixels, based on a matched filter concept and, as an exemplar, design a set of 4 hyperpixels tailored to optimally discriminate between 4 spectral reflectance targets. Next, we fabricate repeating 2x2 pixel filter arrays of these designs, alongside repeating 2x2 arrays of an optimal bandpass filters, perform both spectral and imaging characterization. Experimentally measured hyperpixel transmission spectra show a 2.4x reduction in unmixing matrix condition number (p=0.031) compared to the optimal band-pass set. Imaging experiments using the filter arrays with a monochrome sensor achieve a 3.47x reduction in unmixing matrix condition number (p=0.020) compared to the optimal band-pass set. This demonstrates the utility of the hyperpixel approach and shows its superiority even over the optimal bandpass case. We expect that with further improvements in design and fabrication processes increased performance may be obtained. Because the hyperpixels are straightforward to customize, fabricate and can be placed atop monochrome sensors, this approach is highly versatile and could be adapted to a wide range of real-time imaging applications which are limited by low SNR including micro-endoscopy, capsule endoscopy, industrial inspection and machine vision.
Distribution-informed and wavelength-flexible data-driven photoacoustic oximetry
Mar 21, 2024Abstract:Significance: Photoacoustic imaging (PAI) promises to measure spatially-resolved blood oxygen saturation, but suffers from a lack of accurate and robust spectral unmixing methods to deliver on this promise. Accurate blood oxygenation estimation could have important clinical applications, from cancer detection to quantifying inflammation. Aim: This study addresses the inflexibility of existing data-driven methods for estimating blood oxygenation in PAI by introducing a recurrent neural network architecture. Approach: We created 25 simulated training dataset variations to assess neural network performance. We used a long short-term memory network to implement a wavelength-flexible network architecture and proposed the Jensen-Shannon divergence to predict the most suitable training dataset. Results: The network architecture can handle arbitrary input wavelengths and outperforms linear unmixing and the previously proposed learned spectral decolouring method. Small changes in the training data significantly affect the accuracy of our method, but we find that the Jensen-Shannon divergence correlates with the estimation error and is thus suitable for predicting the most appropriate training datasets for any given application. Conclusions: A flexible data-driven network architecture combined with the Jensen-Shannon Divergence to predict the best training data set provides a promising direction that might enable robust data-driven photoacoustic oximetry for clinical use cases.
Moving beyond simulation: data-driven quantitative photoacoustic imaging using tissue-mimicking phantoms
Jun 11, 2023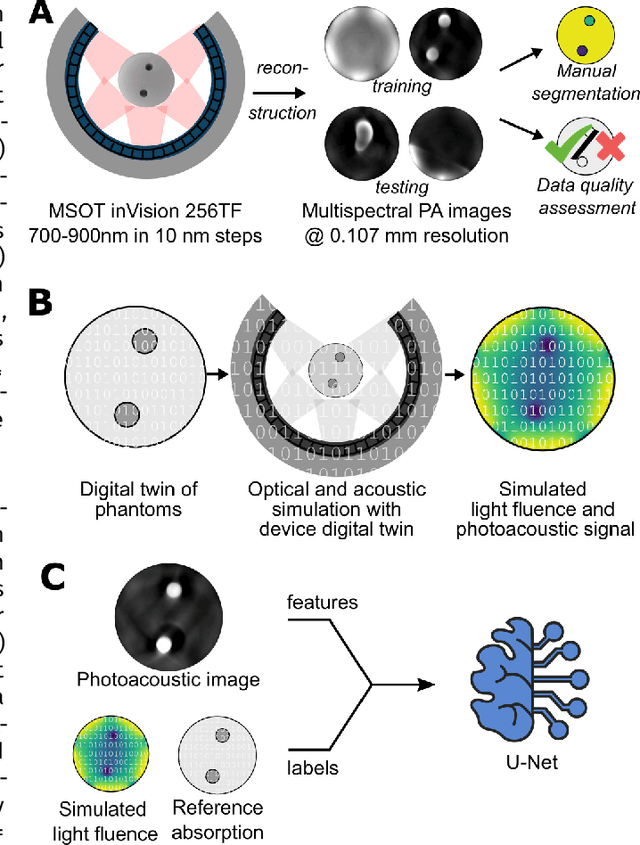
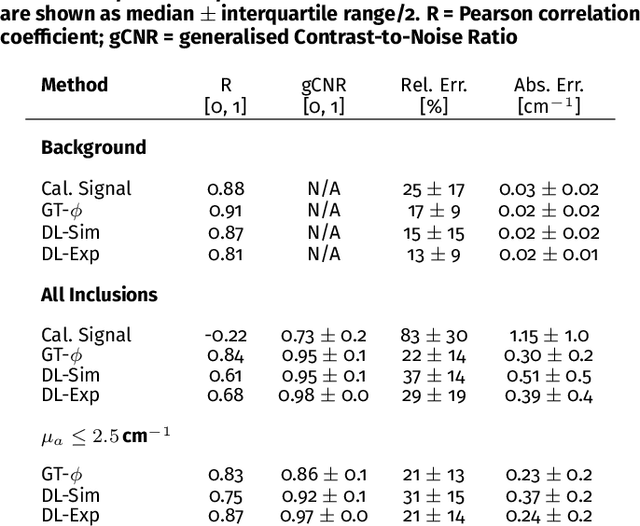
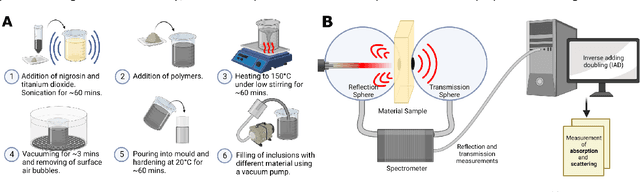
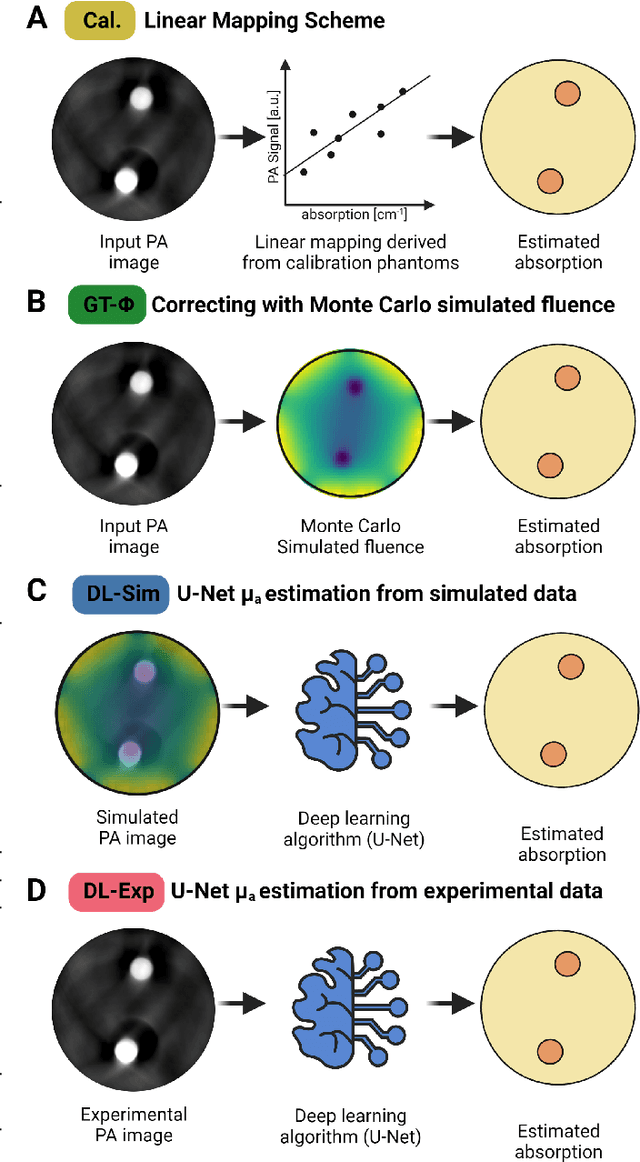
Abstract:Accurate measurement of optical absorption coefficients from photoacoustic imaging (PAI) data would enable direct mapping of molecular concentrations, providing vital clinical insight. The ill-posed nature of the problem of absorption coefficient recovery has prohibited PAI from achieving this goal in living systems due to the domain gap between simulation and experiment. To bridge this gap, we introduce a collection of experimentally well-characterised imaging phantoms and their digital twins. This first-of-a-kind phantom data set enables supervised training of a U-Net on experimental data for pixel-wise estimation of absorption coefficients. We show that training on simulated data results in artefacts and biases in the estimates, reinforcing the existence of a domain gap between simulation and experiment. Training on experimentally acquired data, however, yielded more accurate and robust estimates of optical absorption coefficients. We compare the results to fluence correction with a Monte Carlo model from reference optical properties of the materials, which yields a quantification error of approximately 20%. Application of the trained U-Nets to a blood flow phantom demonstrated spectral biases when training on simulated data, while application to a mouse model highlighted the ability of both learning-based approaches to recover the depth-dependent loss of signal intensity. We demonstrate that training on experimental phantoms can restore the correlation of signal amplitudes measured in depth. While the absolute quantification error remains high and further improvements are needed, our results highlight the promise of deep learning to advance quantitative PAI.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge