Robert Frysch
Institute for Medical Engineering and Research Campus STIMULATE, University of Magdeburg, Magdeburg, Germany
Liver Segmentation in Time-resolved C-arm CT Volumes Reconstructed from Dynamic Perfusion Scans using Time Separation Technique
Feb 09, 2023



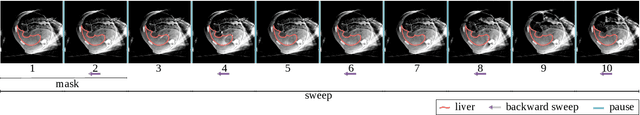
Abstract:Perfusion imaging is a valuable tool for diagnosing and treatment planning for liver tumours. The time separation technique (TST) has been successfully used for modelling C-arm cone-beam computed tomography (CBCT) perfusion data. The reconstruction can be accompanied by the segmentation of the liver - for better visualisation and for generating comprehensive perfusion maps. Recently introduced Turbolift learning has been seen to perform well while working with TST reconstructions, but has not been explored for the time-resolved volumes (TRV) estimated out of TST reconstructions. The segmentation of the TRVs can be useful for tracking the movement of the liver over time. This research explores this possibility by training the multi-scale attention UNet of Turbolift learning at its third stage on the TRVs and shows the robustness of Turbolift learning since it can even work efficiently with the TRVs, resulting in a Dice score of 0.864$\pm$0.004.
Liver Segmentation using Turbolift Learning for CT and Cone-beam C-arm Perfusion Imaging
Jul 20, 2022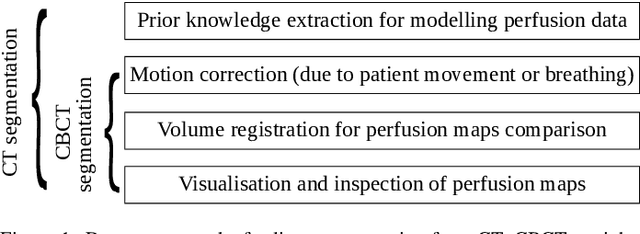
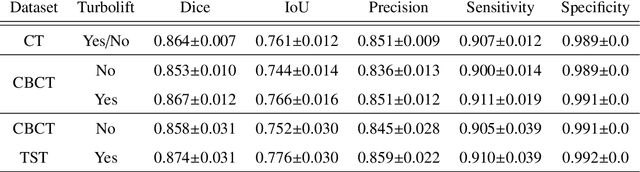
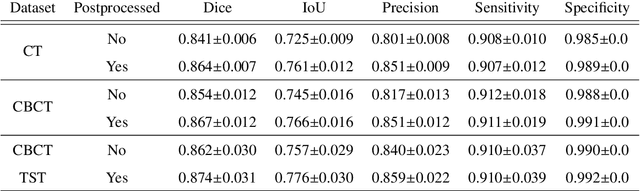
Abstract:Model-based reconstruction employing the time separation technique (TST) was found to improve dynamic perfusion imaging of the liver using C-arm cone-beam computed tomography (CBCT). To apply TST using prior knowledge extracted from CT perfusion data, the liver should be accurately segmented from the CT scans. Reconstructions of primary and model-based CBCT data need to be segmented for proper visualisation and interpretation of perfusion maps. This research proposes Turbolift learning, which trains a modified version of the multi-scale Attention UNet on different liver segmentation tasks serially, following the order of the trainings CT, CBCT, CBCT TST - making the previous trainings act as pre-training stages for the subsequent ones - addressing the problem of limited number of datasets for training. For the final task of liver segmentation from CBCT TST, the proposed method achieved an overall Dice scores of 0.874$\pm$0.031 and 0.905$\pm$0.007 in 6-fold and 4-fold cross-validation experiments, respectively - securing statistically significant improvements over the model, which was trained only for that task. Experiments revealed that Turbolift not only improves the overall performance of the model but also makes it robust against artefacts originating from the embolisation materials and truncation artefacts. Additionally, in-depth analyses confirmed the order of the segmentation tasks. This paper shows the potential of segmenting the liver from CT, CBCT, and CBCT TST, learning from the available limited training data, which can possibly be used in the future for the visualisation and evaluation of the perfusion maps for the treatment evaluation of liver diseases.
Application of Time Separation Technique to Enhance C-arm CT Dynamic Liver Perfusion Imaging
Oct 27, 2021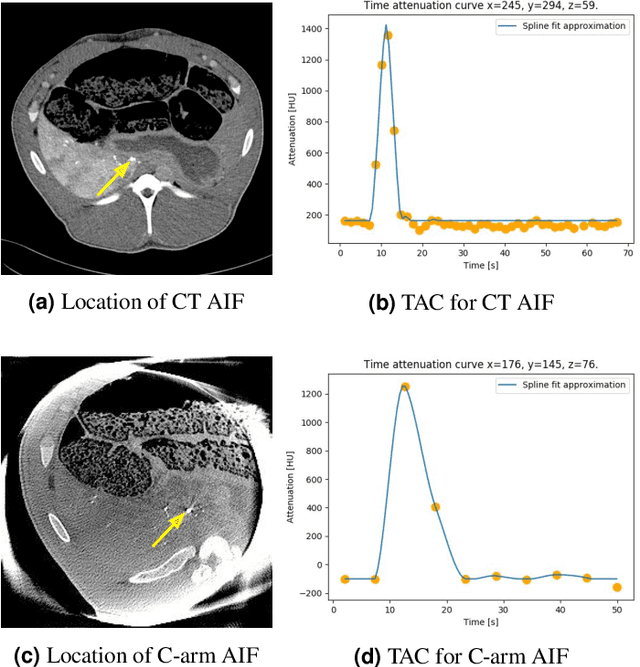
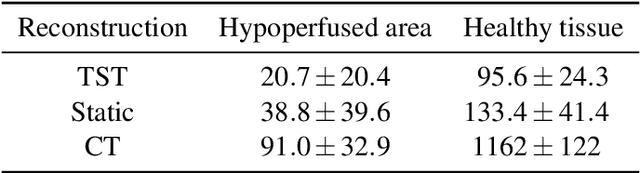
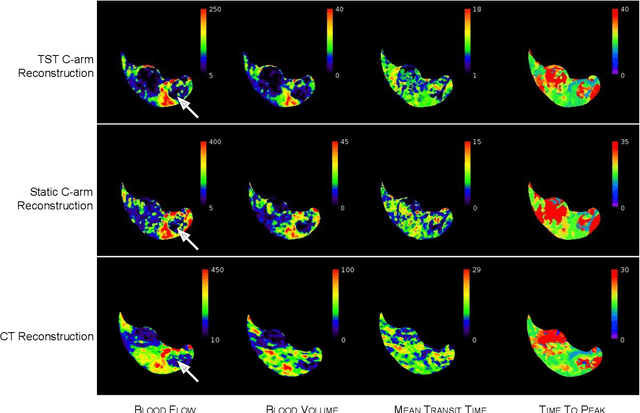
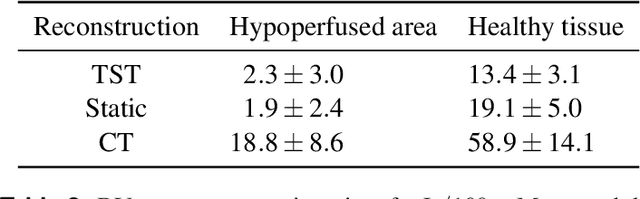
Abstract:Perfusion imaging is an interesting new modality for evaluation and assessment of the liver cancer treatment. C-Arm CT provides a possibility to perform perfusion imaging scans intra-operatively for even faster evaluation. The slow speed of the C-Arm CT rotation and the presence of the noise, however, have an impact on the reconstruction and therefore model based approaches have to be applied. In this work we apply the Time separation technique (TST), to denoise data, speed up reconstruction and improve resulting perfusion images. We show on animal experiment data that Dynamic C-Arm CT Liver Perfusion Imaging together with the processing of the data based on the TST provides comparable results to standard CT liver perfusion imaging.
* 5 pages, 8 figures, published in Proceedings of the 16th Virtual International Meeting on Fully 3D Image Reconstruction in Radiology and Nuclear Medicine 2021 arXiv:2110.04143
Time separation technique with the basis of trigonometric functions as an efficient method for flat detector CT brain perfusion imaging
Oct 18, 2021



Abstract:Dynamic perfusion imaging is routinely used in the diagnostic workup of acute ischemic stroke (AIS). At present, perfusion imaging can also be performed within the angio suite using flat detector computed tomography (FDCT). However, higher noise level, slower rotation speed and lower frame rate need to be considered in FDCT perfusion (FDCTP) data processing algorithms. The Time Separation Technique (TST) is a model-based perfusion data reconstruction method developed to solve these problems. In this contribution, we used TST and dimension reduction, where we approximate the time attenuation curves by a linear combination of trigonometric functions. Our goal was to show that TST with this data reduction does not impair clinical perfusion measurements. We performed a realistic simulation of FDCTP acquisition based on CT perfusion (CTP) data. Using these FDCTP data, we showed that TST provides better results than classical straightforward processing. Moreover we found that TST is robust to additional noise. Furthermore, we achieved a total processing time from reconstruction of FDCTP data to generation of perfusion maps of under 5 minutes. Perfusion maps created using TST with a trigonometric basis from FDCTP data show equivalent perfusion deficits as CT perfusion maps. Therefore, this technique can be considered a fast reliable tool for FDCTP imaging in AIS.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge