Quoc Hung Truong
AdaCS: Adaptive Normalization for Enhanced Code-Switching ASR
Jan 13, 2025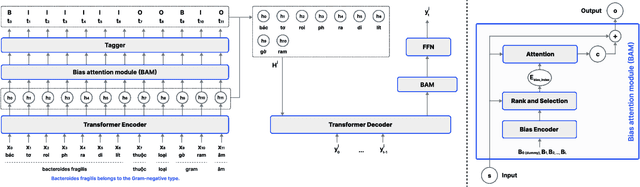
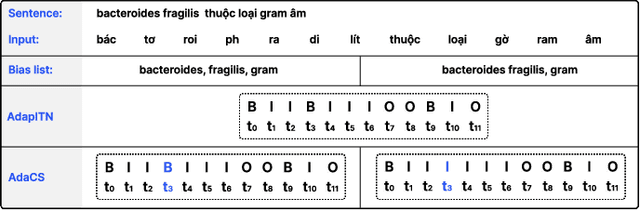
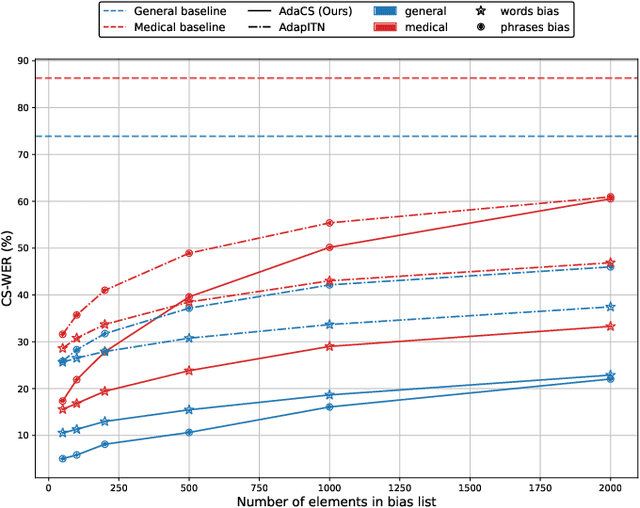

Abstract:Intra-sentential code-switching (CS) refers to the alternation between languages that happens within a single utterance and is a significant challenge for Automatic Speech Recognition (ASR) systems. For example, when a Vietnamese speaker uses foreign proper names or specialized terms within their speech. ASR systems often struggle to accurately transcribe intra-sentential CS due to their training on monolingual data and the unpredictable nature of CS. This issue is even more pronounced for low-resource languages, where limited data availability hinders the development of robust models. In this study, we propose AdaCS, a normalization model integrates an adaptive bias attention module (BAM) into encoder-decoder network. This novel approach provides a robust solution to CS ASR in unseen domains, thereby significantly enhancing our contribution to the field. By utilizing BAM to both identify and normalize CS phrases, AdaCS enhances its adaptive capabilities with a biased list of words provided during inference. Our method demonstrates impressive performance and the ability to handle unseen CS phrases across various domains. Experiments show that AdaCS outperforms previous state-of-the-art method on Vietnamese CS ASR normalization by considerable WER reduction of 56.2% and 36.8% on the two proposed test sets.
RadGraph2: Modeling Disease Progression in Radiology Reports via Hierarchical Information Extraction
Aug 09, 2023



Abstract:We present RadGraph2, a novel dataset for extracting information from radiology reports that focuses on capturing changes in disease state and device placement over time. We introduce a hierarchical schema that organizes entities based on their relationships and show that using this hierarchy during training improves the performance of an information extraction model. Specifically, we propose a modification to the DyGIE++ framework, resulting in our model HGIE, which outperforms previous models in entity and relation extraction tasks. We demonstrate that RadGraph2 enables models to capture a wider variety of findings and perform better at relation extraction compared to those trained on the original RadGraph dataset. Our work provides the foundation for developing automated systems that can track disease progression over time and develop information extraction models that leverage the natural hierarchy of labels in the medical domain.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge