Peter R. Mouton
Active Prompt Tuning Enables Gpt-40 To Do Efficient Classification Of Microscopy Images
Nov 04, 2024Abstract:Traditional deep learning-based methods for classifying cellular features in microscopy images require time- and labor-intensive processes for training models. Among the current limitations are major time commitments from domain experts for accurate ground truth preparation; and the need for a large amount of input image data. We previously proposed a solution that overcomes these challenges using OpenAI's GPT-4(V) model on a pilot dataset (Iba-1 immuno-stained tissue sections from 11 mouse brains). Results on the pilot dataset were equivalent in accuracy and with a substantial improvement in throughput efficiency compared to the baseline using a traditional Convolutional Neural Net (CNN)-based approach. The present study builds upon this framework using a second unique and substantially larger dataset of microscopy images. Our current approach uses a newer and faster model, GPT-4o, along with improved prompts. It was evaluated on a microscopy image dataset captured at low (10x) magnification from cresyl-violet-stained sections through the cerebellum of a total of 18 mouse brains (9 Lurcher mice, 9 wild-type controls). We used our approach to classify these images either as a control group or Lurcher mutant. Using 6 mice in the prompt set the results were correct classification for 11 out of the 12 mice (92%) with 96% higher efficiency, reduced image requirements, and lower demands on time and effort of domain experts compared to the baseline method (snapshot ensemble of CNN models). These results confirm that our approach is effective across multiple datasets from different brain regions and magnifications, with minimal overhead.
Iterative Deep Learning Based Unbiased Stereology With Human-in-the-Loop
Jan 14, 2019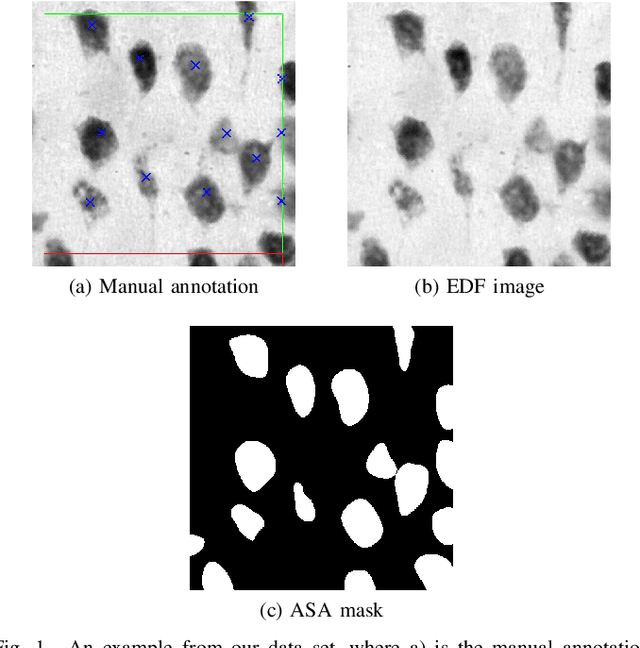
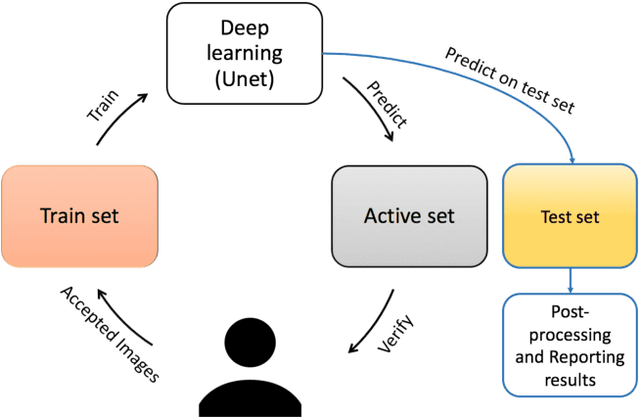
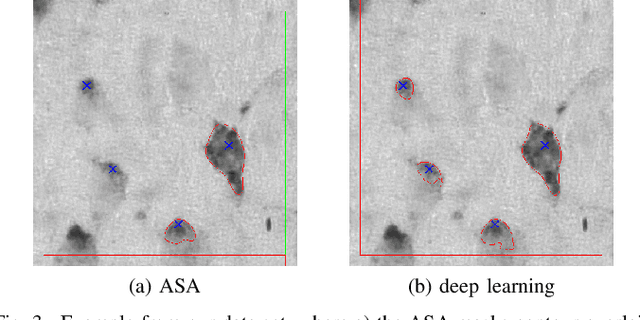
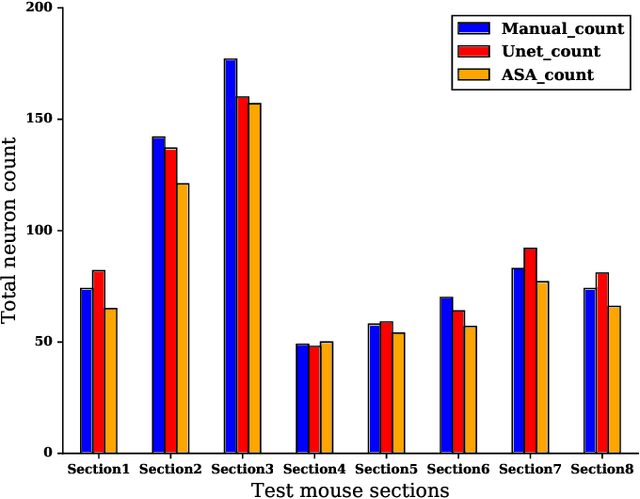
Abstract:Lack of enough labeled data is a major problem in building machine learning based models when the manual annotation (labeling) is error-prone, expensive, tedious, and time-consuming. In this paper, we introduce an iterative deep learning based method to improve segmentation and counting of cells based on unbiased stereology applied to regions of interest of extended depth of field (EDF) images. This method uses an existing machine learning algorithm called the adaptive segmentation algorithm (ASA) to generate masks (verified by a user) for EDF images to train deep learning models. Then an iterative deep learning approach is used to feed newly predicted and accepted deep learning masks/images (verified by a user) to the training set of the deep learning model. The error rate in unbiased stereology count of cells on an unseen test set reduced from about 3 % to less than 1 % after 5 iterations of the iterative deep learning based unbiased stereology process.
A New Cervical Cytology Dataset for Nucleus Detection and Image Classification and Methods for Cervical Nucleus Detection
Nov 23, 2018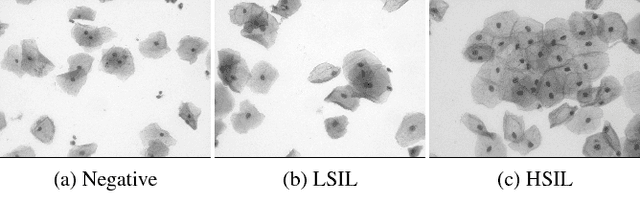

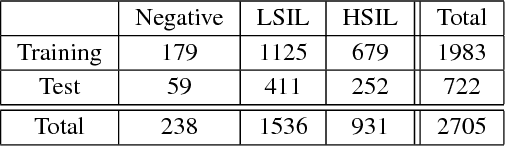
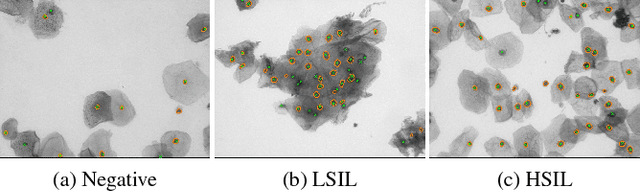
Abstract:Analyzing Pap cytology slides is an important tasks in detecting and grading precancerous and cancerous cervical cancer stages. Processing cytology images usually involve segmenting nuclei and overlapping cells. We introduce a cervical cytology dataset that can be used to evaluate nucleus detection, as well as image classification methods in the cytology image processing area. This dataset contains 93 real image stacks with their grade labels and manually annotated nuclei within images. We also present two methods: a baseline method based on a previously proposed approach, and a deep learning method, and compare their results with other state-of-the-art methods. Both the baseline method and the deep learning method outperform other state-of-the-art methods by significant margins. Along with the dataset, we publicly make the evaluation code and the baseline method available to download for further benchmarking.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge