Peijie Zhou
WFR-MFM: One-Step Inference for Dynamic Unbalanced Optimal Transport
Jan 28, 2026Abstract:Reconstructing dynamical evolution from limited observations is a fundamental challenge in single-cell biology, where dynamic unbalanced optimal transport provides a principled framework for modeling coupled transport and mass variation. However, existing approaches rely on trajectory simulation at inference time, making inference a key bottleneck for scalable applications. In this work, we propose a mean-flow framework for unbalanced flow matching that summarizes both transport and mass-growth dynamics over arbitrary time intervals using mean velocity and mass-growth fields, enabling fast one-step generation without trajectory simulation. To solve dynamic unbalanced optimal transport under the Wasserstein-Fisher-Rao geometry, we further build on this framework to develop Wasserstein-Fisher-Rao Mean Flow Matching (WFR-MFM). Across synthetic and real single-cell RNA sequencing datasets, WFR-MFM achieves orders-of-magnitude faster inference than a range of existing baselines while maintaining high predictive accuracy, and enables efficient perturbation response prediction on large synthetic datasets with thousands of conditions.
WFR-FM: Simulation-Free Dynamic Unbalanced Optimal Transport
Jan 11, 2026Abstract:The Wasserstein-Fisher-Rao (WFR) metric extends dynamic optimal transport (OT) by coupling displacement with change of mass, providing a principled geometry for modeling unbalanced snapshot dynamics. Existing WFR solvers, however, are often unstable, computationally expensive, and difficult to scale. Here we introduce WFR Flow Matching (WFR-FM), a simulation-free training algorithm that unifies flow matching with dynamic unbalanced OT. Unlike classical flow matching which regresses only a transport vector field, WFR-FM simultaneously regresses a vector field for displacement and a scalar growth rate function for birth-death dynamics, yielding continuous flows under the WFR geometry. Theoretically, we show that minimizing the WFR-FM loss exactly recovers WFR geodesics. Empirically, WFR-FM yields more accurate and robust trajectory inference in single-cell biology, reconstructing consistent dynamics with proliferation and apoptosis, estimating time-varying growth fields, and applying to generative dynamics under imbalanced data. It outperforms state-of-the-art baselines in efficiency, stability, and reconstruction accuracy. Overall, WFR-FM establishes a unified and efficient paradigm for learning dynamical systems from unbalanced snapshots, where not only states but also mass evolve over time.
CellStream: Dynamical Optimal Transport Informed Embeddings for Reconstructing Cellular Trajectories from Snapshots Data
Nov 16, 2025Abstract:Single-cell RNA sequencing (scRNA-seq), especially temporally resolved datasets, enables genome-wide profiling of gene expression dynamics at single-cell resolution across discrete time points. However, current technologies provide only sparse, static snapshots of cell states and are inherently influenced by technical noise, complicating the inference and representation of continuous transcriptional dynamics. Although embedding methods can reduce dimensionality and mitigate technical noise, the majority of existing approaches typically treat trajectory inference separately from embedding construction, often neglecting temporal structure. To address this challenge, here we introduce CellStream, a novel deep learning framework that jointly learns embedding and cellular dynamics from single-cell snapshot data by integrating an autoencoder with unbalanced dynamical optimal transport. Compared to existing methods, CellStream generates dynamics-informed embeddings that robustly capture temporal developmental processes while maintaining high consistency with the underlying data manifold. We demonstrate CellStream's effectiveness on both simulated datasets and real scRNA-seq data, including spatial transcriptomics. Our experiments indicate significant quantitative improvements over state-of-the-art methods in representing cellular trajectories with enhanced temporal coherence and reduced noise sensitivity. Overall, CellStream provides a new tool for learning and representing continuous streams from the noisy, static snapshots of single-cell gene expression.
Joint Velocity-Growth Flow Matching for Single-Cell Dynamics Modeling
May 19, 2025



Abstract:Learning the underlying dynamics of single cells from snapshot data has gained increasing attention in scientific and machine learning research. The destructive measurement technique and cell proliferation/death result in unpaired and unbalanced data between snapshots, making the learning of the underlying dynamics challenging. In this paper, we propose joint Velocity-Growth Flow Matching (VGFM), a novel paradigm that jointly learns state transition and mass growth of single-cell populations via flow matching. VGFM builds an ideal single-cell dynamics containing velocity of state and growth of mass, driven by a presented two-period dynamic understanding of the static semi-relaxed optimal transport, a mathematical tool that seeks the coupling between unpaired and unbalanced data. To enable practical usage, we approximate the ideal dynamics using neural networks, forming our joint velocity and growth matching framework. A distribution fitting loss is also employed in VGFM to further improve the fitting performance for snapshot data. Extensive experimental results on both synthetic and real datasets demonstrate that VGFM can capture the underlying biological dynamics accounting for mass and state variations over time, outperforming existing approaches for single-cell dynamics modeling.
Variational Regularized Unbalanced Optimal Transport: Single Network, Least Action
May 17, 2025Abstract:Recovering the dynamics from a few snapshots of a high-dimensional system is a challenging task in statistical physics and machine learning, with important applications in computational biology. Many algorithms have been developed to tackle this problem, based on frameworks such as optimal transport and the Schr\"odinger bridge. A notable recent framework is Regularized Unbalanced Optimal Transport (RUOT), which integrates both stochastic dynamics and unnormalized distributions. However, since many existing methods do not explicitly enforce optimality conditions, their solutions often struggle to satisfy the principle of least action and meet challenges to converge in a stable and reliable way. To address these issues, we propose Variational RUOT (Var-RUOT), a new framework to solve the RUOT problem. By incorporating the optimal necessary conditions for the RUOT problem into both the parameterization of the search space and the loss function design, Var-RUOT only needs to learn a scalar field to solve the RUOT problem and can search for solutions with lower action. We also examined the challenge of selecting a growth penalty function in the widely used Wasserstein-Fisher-Rao metric and proposed a solution that better aligns with biological priors in Var-RUOT. We validated the effectiveness of Var-RUOT on both simulated data and real single-cell datasets. Compared with existing algorithms, Var-RUOT can find solutions with lower action while exhibiting faster convergence and improved training stability.
Modeling Cell Dynamics and Interactions with Unbalanced Mean Field Schrödinger Bridge
May 16, 2025Abstract:Modeling the dynamics from sparsely time-resolved snapshot data is crucial for understanding complex cellular processes and behavior. Existing methods leverage optimal transport, Schr\"odinger bridge theory, or their variants to simultaneously infer stochastic, unbalanced dynamics from snapshot data. However, these approaches remain limited in their ability to account for cell-cell interactions. This integration is essential in real-world scenarios since intercellular communications are fundamental life processes and can influence cell state-transition dynamics. To address this challenge, we formulate the Unbalanced Mean-Field Schr\"odinger Bridge (UMFSB) framework to model unbalanced stochastic interaction dynamics from snapshot data. Inspired by this framework, we further propose CytoBridge, a deep learning algorithm designed to approximate the UMFSB problem. By explicitly modeling cellular transitions, proliferation, and interactions through neural networks, CytoBridge offers the flexibility to learn these processes directly from data. The effectiveness of our method has been extensively validated using both synthetic gene regulatory data and real scRNA-seq datasets. Compared to existing methods, CytoBridge identifies growth, transition, and interaction patterns, eliminates false transitions, and reconstructs the developmental landscape with greater accuracy.
Integrating Dynamical Systems Modeling with Spatiotemporal scRNA-seq Data Analysis
Mar 14, 2025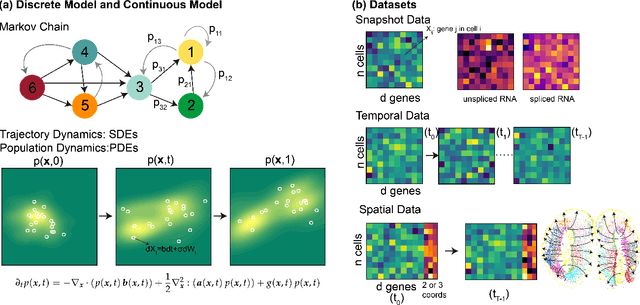
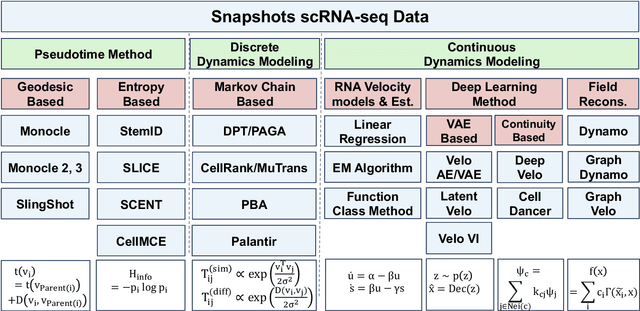
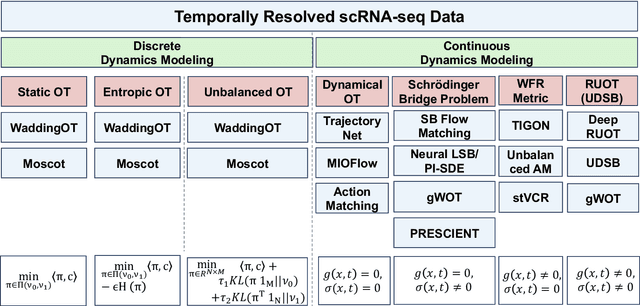
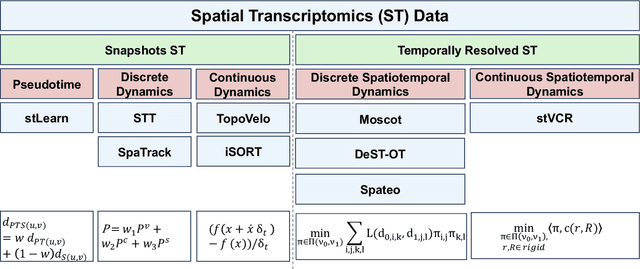
Abstract:Understanding the dynamic nature of biological systems is fundamental to deciphering cellular behavior, developmental processes, and disease progression. Single-cell RNA sequencing (scRNA-seq) has provided static snapshots of gene expression, offering valuable insights into cellular states at a single time point. Recent advancements in temporally resolved scRNA-seq, spatial transcriptomics (ST), and time-series spatial transcriptomics (temporal-ST) have further revolutionized our ability to study the spatiotemporal dynamics of individual cells. These technologies, when combined with computational frameworks such as Markov chains, stochastic differential equations (SDEs), and generative models like optimal transport and Schr\"odinger bridges, enable the reconstruction of dynamic cellular trajectories and cell fate decisions. This review discusses how these dynamical system approaches offer new opportunities to model and infer cellular dynamics from a systematic perspective.
Learning Stochastic Dynamics from Snapshots through Regularized Unbalanced Optimal Transport
Oct 01, 2024Abstract:Reconstructing dynamics using samples from sparsely time-resolved snapshots is an important problem in both natural sciences and machine learning. Here, we introduce a new deep learning approach for solving regularized unbalanced optimal transport (RUOT) and inferring continuous unbalanced stochastic dynamics from observed snapshots. Based on the RUOT form, our method models these dynamics without requiring prior knowledge of growth and death processes or additional information, allowing them to be learnt directly from data. Theoretically, we explore the connections between the RUOT and Schr\"odinger bridge problem and discuss the key challenges and potential solutions. The effectiveness of our method is demonstrated with a synthetic gene regulatory network. Compared with other methods, our approach accurately identifies growth and transition patterns, eliminates false transitions, and constructs the Waddington developmental landscape.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge