Oliver Blanck
FedSynthCT-Brain: A Federated Learning Framework for Multi-Institutional Brain MRI-to-CT Synthesis
Dec 09, 2024



Abstract:The generation of Synthetic Computed Tomography (sCT) images has become a pivotal methodology in modern clinical practice, particularly in the context of Radiotherapy (RT) treatment planning. The use of sCT enables the calculation of doses, pushing towards Magnetic Resonance Imaging (MRI) guided radiotherapy treatments. Moreover, with the introduction of MRI-Positron Emission Tomography (PET) hybrid scanners, the derivation of sCT from MRI can improve the attenuation correction of PET images. Deep learning methods for MRI-to-sCT have shown promising results, but their reliance on single-centre training dataset limits generalisation capabilities to diverse clinical settings. Moreover, creating centralised multicentre datasets may pose privacy concerns. To solve the issues, this study introduces FedSynthCT-Brain, a framework based on the Federated Learning (FL) paradigm for MRI-to-sCT in brain imaging. We reproduced a federation across four European and American centres using a U-Net-based model. The approach was implemented using data from centres belonging the federation and it was tested on an unseen dataset from a centre outside the federation. In the case of the unseen centre, the federated model achieved a median Mean Absolute Error (MAE) of 102.0 HU across 23 patients, with an interquartile range of 96.7-110.5 HU. The median (interquartile range) for the Structural Similarity Index (SSIM) and the Peak Signal to Noise Ratio (PNSR) were 0.89 (0.86-0.89) and 26.58 (25.52-27.42), respectively. The analysis of the results showed acceptable performances of the federated approach, thus highlighting the potential of FL to enhance MRI-to-sCT to improve generalisability and advancing safe and equitable clinical applications while fostering collaboration and preserving data privacy.
Analysis of clinical, dosimetric and radiomic features for predicting local failure after stereotactic radiotherapy of brain metastases in malignant melanoma
May 31, 2024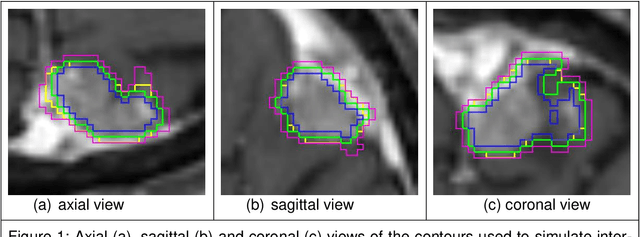
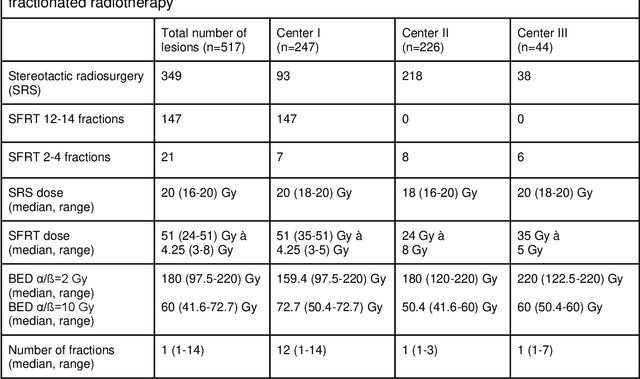
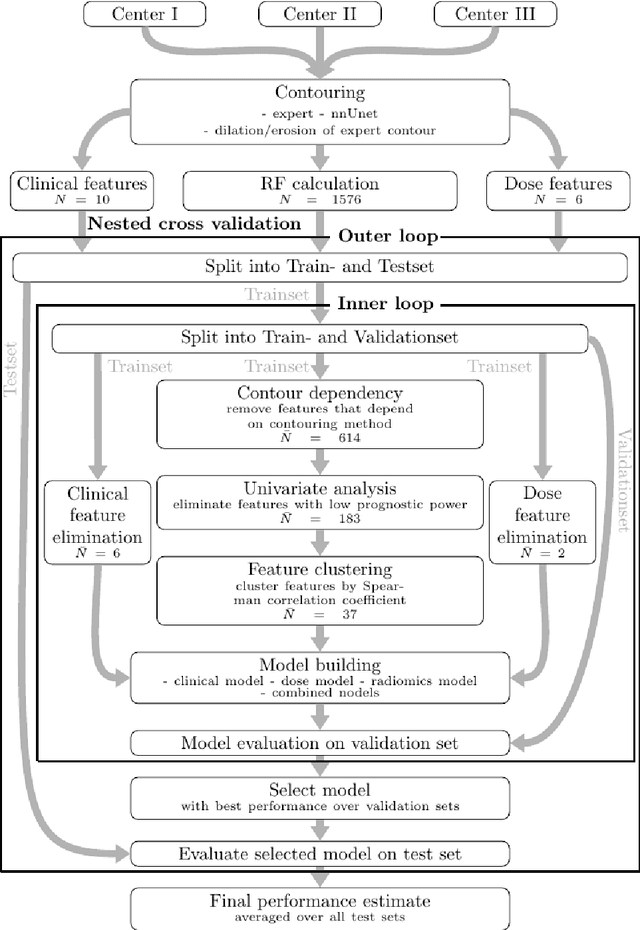
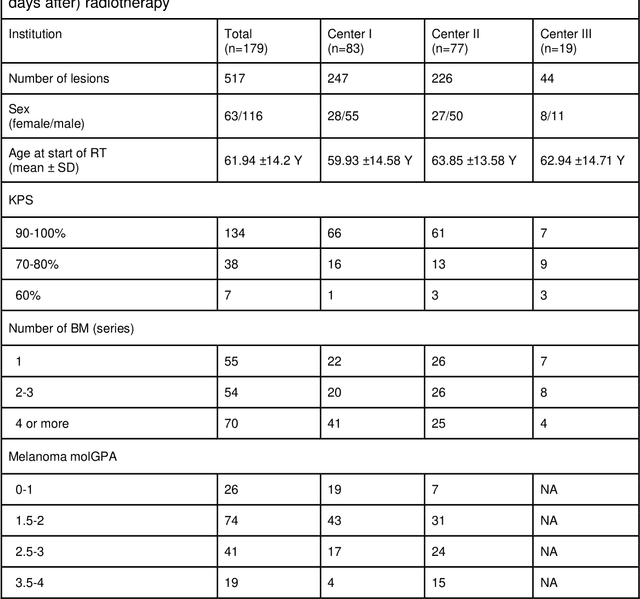
Abstract:Background: The aim of this study was to investigate the role of clinical, dosimetric and pretherapeutic magnetic resonance imaging (MRI) features for lesion-specific outcome prediction of stereotactic radiotherapy (SRT) in patients with brain metastases from malignant melanoma (MBM). Methods: In this multicenter, retrospective analysis, we reviewed 517 MBM from 130 patients treated with SRT (single fraction or hypofractionated). For each gross tumor volume (GTV) 1576 radiomic features (RF) were calculated (788 each for the GTV and for a 3 mm margin around the GTV). Clinical parameters, radiation dose and RF from pretherapeutic contrast-enhanced T1-weighted MRI from different institutions were evaluated with a feature processing and elimination pipeline in a nested cross-validation scheme. Results: Seventy-two (72) of 517 lesions (13.9%) showed a local failure (LF) after SRT. The processing pipeline showed clinical, dosimetric and radiomic features providing information for LF prediction. The most prominent ones were the correlation of the gray level co-occurrence matrix of the margin (hazard ratio (HR): 0.37, confidence interval (CI): 0.23-0.58) and systemic therapy before SRT (HR: 0.55, CI: 0.42-0.70). The majority of RF associated with LF was calculated in the margin around the GTV. Conclusions: Pretherapeutic MRI based RF connected with lesion-specific outcome after SRT could be identified, despite multicentric data and minor differences in imaging protocols. Image data analysis of the surrounding metastatic environment may provide therapy-relevant information with the potential to further individualize radiotherapy strategies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge