Ilias Sachpazidis
Analysis of clinical, dosimetric and radiomic features for predicting local failure after stereotactic radiotherapy of brain metastases in malignant melanoma
May 31, 2024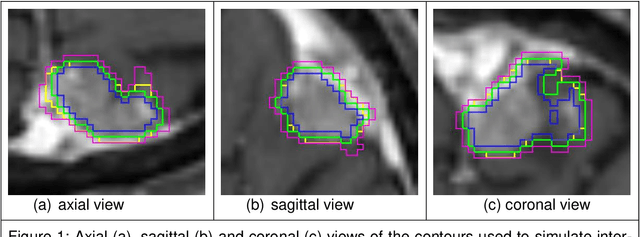
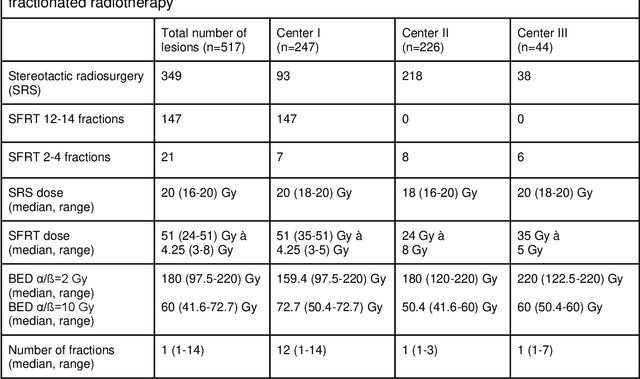
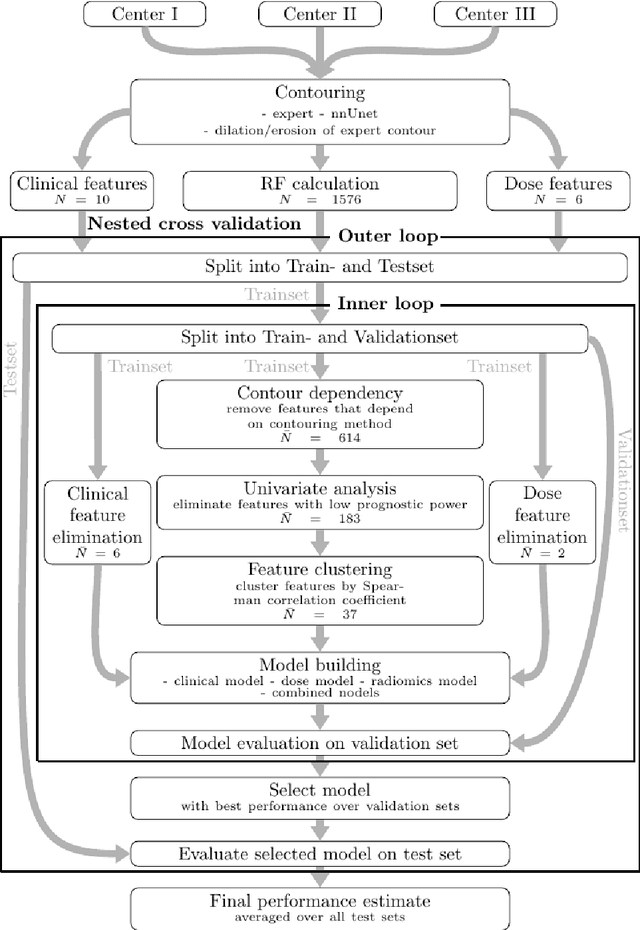
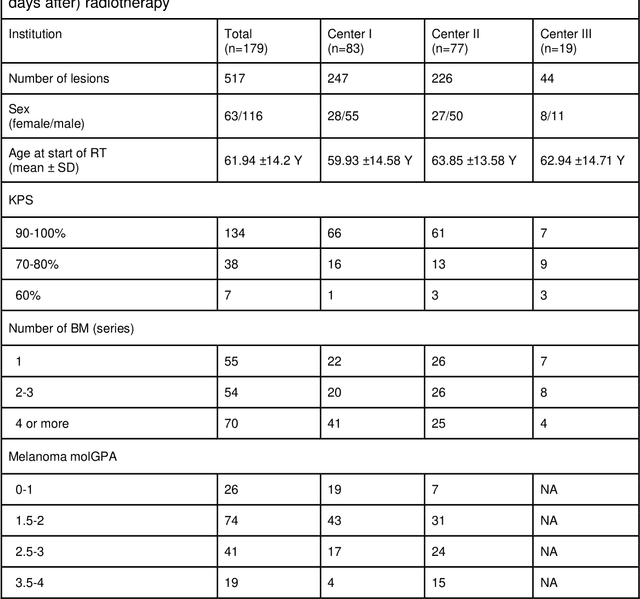
Abstract:Background: The aim of this study was to investigate the role of clinical, dosimetric and pretherapeutic magnetic resonance imaging (MRI) features for lesion-specific outcome prediction of stereotactic radiotherapy (SRT) in patients with brain metastases from malignant melanoma (MBM). Methods: In this multicenter, retrospective analysis, we reviewed 517 MBM from 130 patients treated with SRT (single fraction or hypofractionated). For each gross tumor volume (GTV) 1576 radiomic features (RF) were calculated (788 each for the GTV and for a 3 mm margin around the GTV). Clinical parameters, radiation dose and RF from pretherapeutic contrast-enhanced T1-weighted MRI from different institutions were evaluated with a feature processing and elimination pipeline in a nested cross-validation scheme. Results: Seventy-two (72) of 517 lesions (13.9%) showed a local failure (LF) after SRT. The processing pipeline showed clinical, dosimetric and radiomic features providing information for LF prediction. The most prominent ones were the correlation of the gray level co-occurrence matrix of the margin (hazard ratio (HR): 0.37, confidence interval (CI): 0.23-0.58) and systemic therapy before SRT (HR: 0.55, CI: 0.42-0.70). The majority of RF associated with LF was calculated in the margin around the GTV. Conclusions: Pretherapeutic MRI based RF connected with lesion-specific outcome after SRT could be identified, despite multicentric data and minor differences in imaging protocols. Image data analysis of the surrounding metastatic environment may provide therapy-relevant information with the potential to further individualize radiotherapy strategies.
The use of deep learning in interventional radiotherapy : a review with a focus on open source and open data
May 16, 2022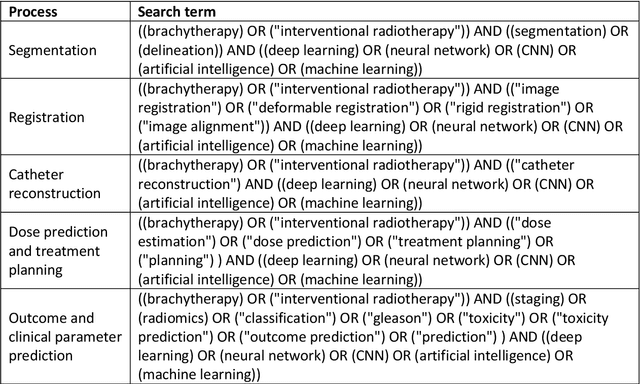
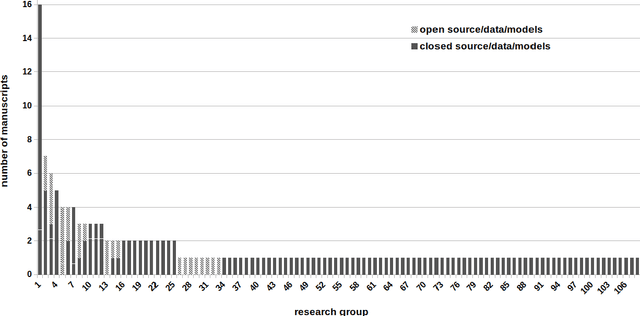
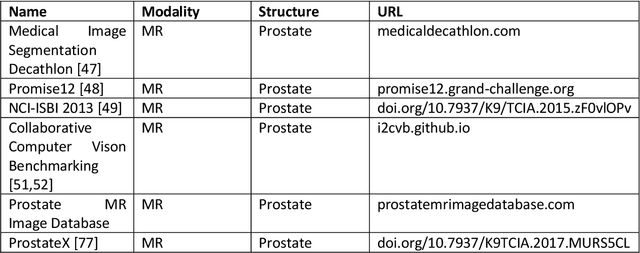
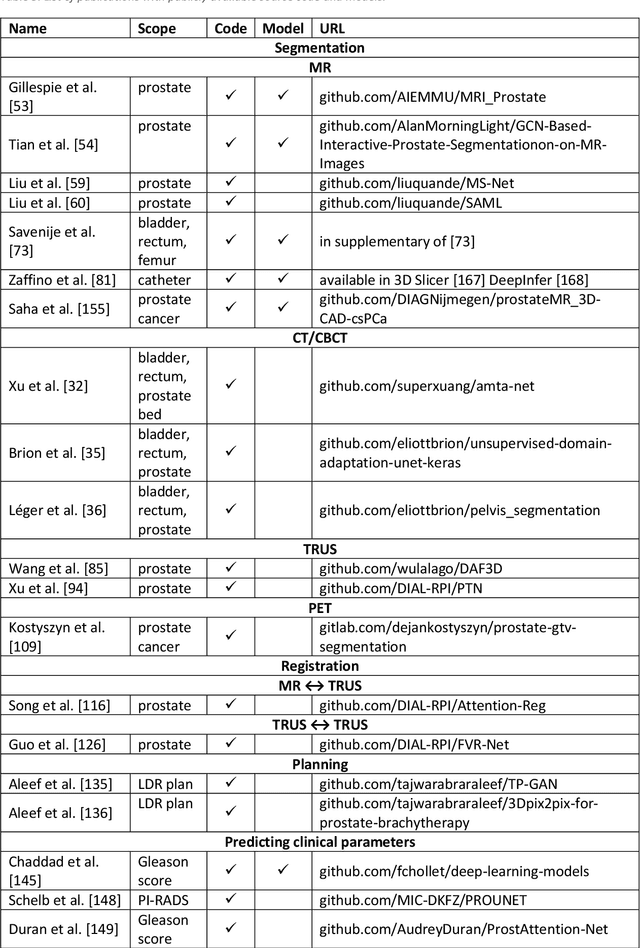
Abstract:Deep learning advanced to one of the most important technologies in almost all medical fields. Especially in areas, related to medical imaging it plays a big role. However, in interventional radiotherapy (brachytherapy) deep learning is still in an early phase. In this review, first, we investigated and scrutinised the role of deep learning in all processes of interventional radiotherapy and directly related fields. Additionally we summarised the most recent developments. To reproduce results of deep learning algorithms both source code and training data must be available. Therefore, a second focus of this work was on the analysis of the availability of open source, open data and open models. In our analysis, we were able to show that deep learning plays already a major role in some areas of interventional radiotherapy, but is still hardly presented in others. Nevertheless, its impact is increasing with the years, partly self-propelled but also influenced by closely related fields. Open source, data and models are growing in number but are still scarce and unevenly distributed among different research groups. The reluctance in publishing code, data and models limits reproducibility and restricts evaluation to mono-institutional datasets. Summarised, deep learning will change positively the workflow of interventional radiotherapy but there is room for improvement when it comes to reproducible results and standardised evaluation methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge