Nick Bottenus
Optimization of Array Encoding for Ultrasound Imaging
Mar 01, 2024Abstract:Objective: The transmit encoding model for synthetic aperture imaging is a robust and flexible framework for understanding the effect of acoustic transmission on ultrasound image reconstruction. Our objective is to use machine learning (ML) to construct scanning sequences, parameterized by time delays and apodization weights, that produce high quality B-mode images. Approach: We use an ML model in PyTorch and simulated RF data from Field II to probe the space of possible encoding sequences for those that minimize a loss function that describes image quality. This approach is made computationally feasible by a novel formulation of the derivative for delay-and-sum beamforming. We demonstrate these results experimentally on wire targets and a tissue-mimicking phantom. Main Results: When trained according to a given set of imaging parameters (imaging domain, hardware restrictions), our ML imaging model produces optimized encoding sequences that improve a number of standard quality metrics including resolution, field of view, and contrast, over conventional sequences. Significance: This work demonstrates that the set of encoding schemes that are commonly used represent only a narrow subset of those available. Additionally, it demonstrates the value for ML tasks in synthetic transmit aperture imaging to consider the beamformer within the model, instead of as purely post-processing.
MimickNet, Matching Clinical Post-Processing Under Realistic Black-Box Constraints
Aug 15, 2019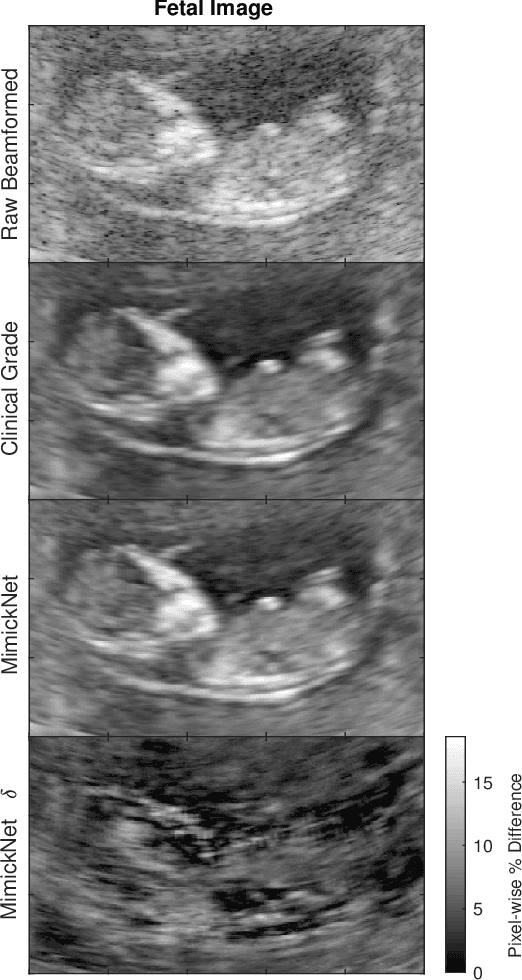
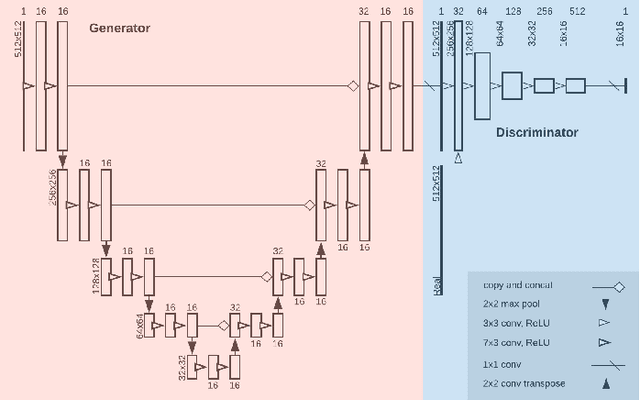
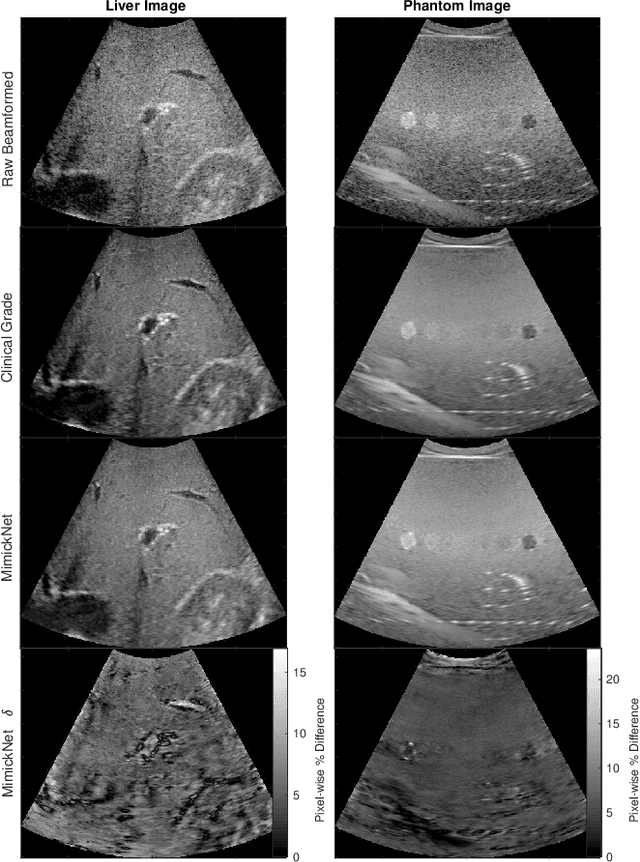
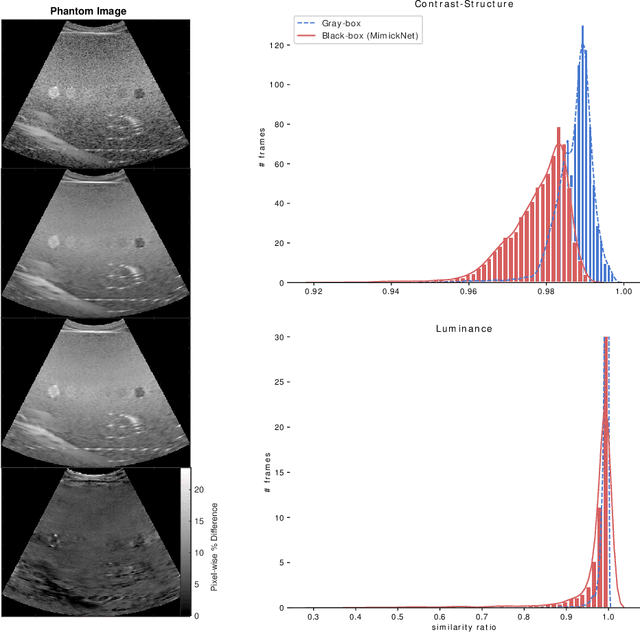
Abstract:Image post-processing is used in clinical-grade ultrasound scanners to improve image quality (e.g., reduce speckle noise and enhance contrast). These post-processing techniques vary across manufacturers and are generally kept proprietary, which presents a challenge for researchers looking to match current clinical-grade workflows. We introduce a deep learning framework, MimickNet, that transforms raw conventional delay-and-summed (DAS) beams into the approximate post-processed images found on clinical-grade scanners. Training MimickNet only requires post-processed image samples from a scanner of interest without the need for explicit pairing to raw DAS data. This flexibility allows it to hypothetically approximate any manufacturer's post-processing without access to the pre-processed data. MimickNet generates images with an average similarity index measurement (SSIM) of 0.930$\pm$0.0892 on a 300 cineloop test set, and it generalizes to cardiac cineloops outside of our train-test distribution achieving an SSIM of 0.967$\pm$0.002. We also explore the theoretical SSIM achievable by evaluating MimickNet performance when trained under gray-box constraints (i.e., when both pre-processed and post-processed images are available). To our knowledge, this is the first work to establish deep learning models that closely approximate current clinical-grade ultrasound post-processing under realistic black-box constraints where before and after post-processing data is unavailable. MimickNet serves as a clinical post-processing baseline for future works in ultrasound image formation to compare against. To this end, we have made the MimickNet software open source.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge