Naoki Tomii
Online camera-pose-free stereo endoscopic tissue deformation recovery with tissue-invariant vision-biomechanics consistency
Jun 24, 2025Abstract:Tissue deformation recovery based on stereo endoscopic images is crucial for tool-tissue interaction analysis and benefits surgical navigation and autonomous soft tissue manipulation. Previous research suffers from the problems raised from camera motion, occlusion, large tissue deformation, lack of tissue-specific biomechanical priors, and reliance on offline processing. Unlike previous studies where the tissue geometry and deformation are represented by 3D points and displacements, the proposed method models tissue geometry as the 3D point and derivative map and tissue deformation as the 3D displacement and local deformation map. For a single surface point, 6 parameters are used to describe its rigid motion and 3 parameters for its local deformation. The method is formulated under the camera-centric setting, where all motions are regarded as the scene motion with respect to the camera. Inter-frame alignment is realized by optimizing the inter-frame deformation, making it unnecessary to estimate camera pose. The concept of the canonical map is introduced to optimize tissue geometry and deformation in an online approach. Quantitative and qualitative experiments were conducted using in vivo and ex vivo laparoscopic datasets. With the inputs of depth and optical flow, the method stably models tissue geometry and deformation even when the tissue is partially occluded or moving outside the field of view. Results show that the 3D reconstruction accuracy in the non-occluded and occluded areas reaches 0.37$\pm$0.27 mm and 0.39$\pm$0.21 mm in terms of surface distance, respectively. The method can also estimate surface strain distribution during various manipulations as an extra modality for mechanical-based analysis.
Deep-learning-based electrode action potential mapping (DEAP Mapping) from annotation-free unipolar electrogram
Aug 07, 2024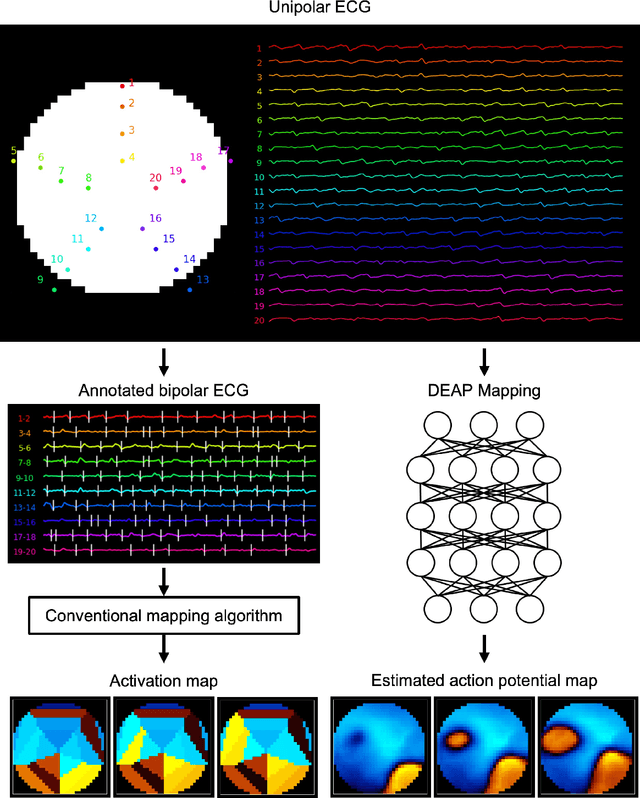
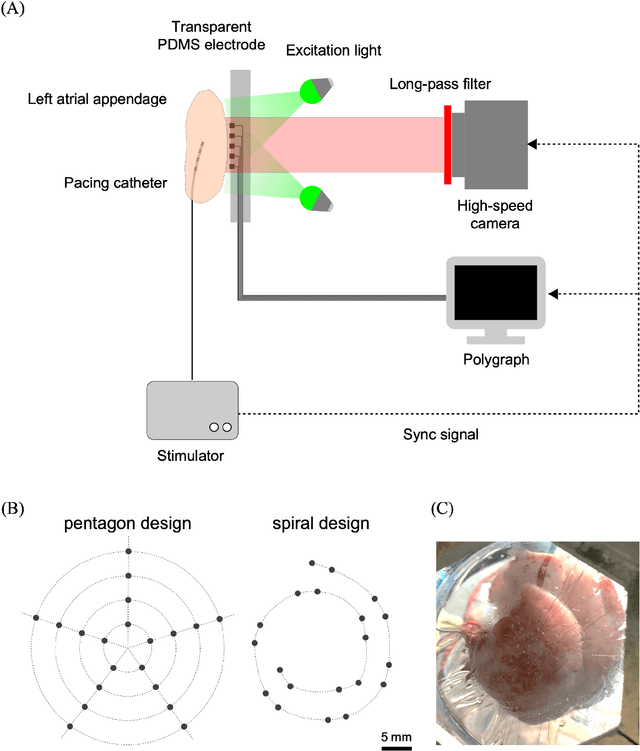
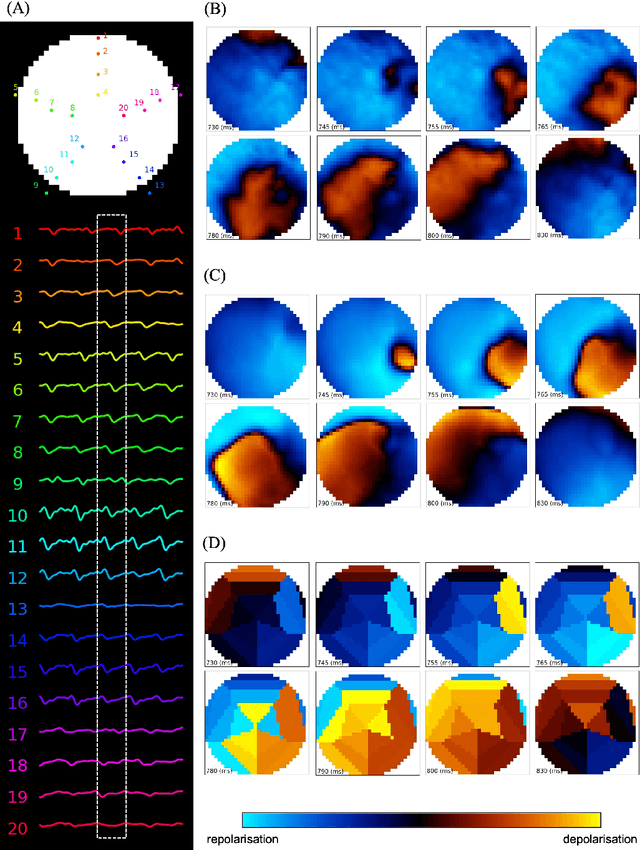
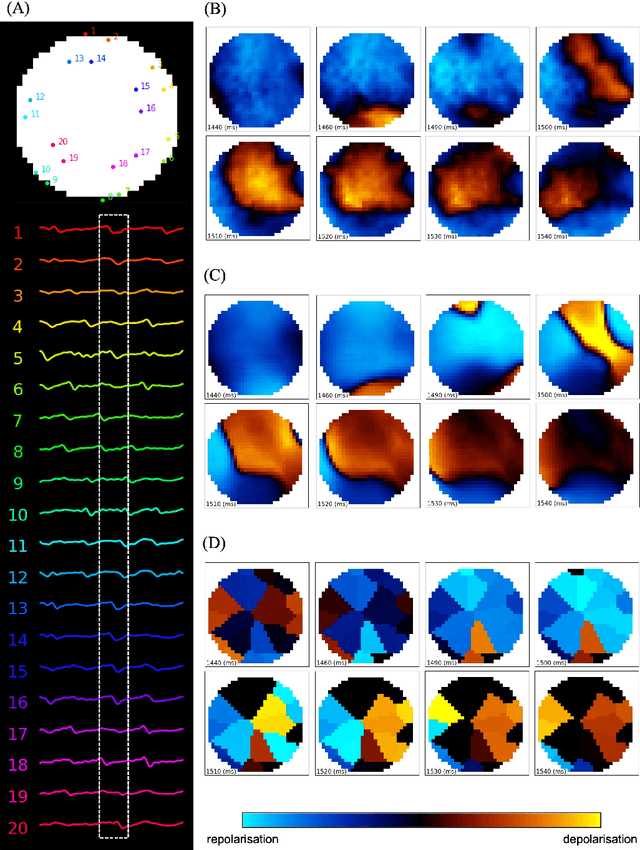
Abstract:Catheter ablation has limited therapeutic efficacy against non-paroxysmal atrial fibrillation (AF), and electrophysiological studies using mapping catheters have been applied to evaluate the AF substrate. However, many of these approaches rely on detecting excitation timing from electrograms (ECGs), potentially compromising their effectiveness in complex AF scenarios. Herein, we introduce Deep-learning-based Electrode Action Potential Mapping (DEAP Mapping), a deep learning model designed to reconstruct membrane potential images from annotation-free unipolar ECG signals. We conducted ex vivo experiments using porcine hearts (N = 6) to evaluate the accuracy of DEAP Mapping by simultaneously performing fluorescence measurement of membrane potentials and measurements of epicardial unipolar ECGs. Membrane potentials estimated via DEAP Mapping were compared with those measured via optical mapping. We assessed the clinical applicability of DEAP Mapping by comparing the DEAP Mapping's estimations from clinically measured catheter electrode signals with those from established electrode-mapping techniques. DEAP Mapping accurately estimated conduction delays and blocks in ex vivo experiments. Phase variance analysis, an AF substrate evaluation method, revealed that the substrate identified from optical mapping closely resembled that identified from DEAP Mapping estimations (structural similarity index of >0.8). In clinical evaluations, DEAP Mapping estimation observed several conduction delays and blocks that were not observed with existing methods, indicating that DEAP Mapping can estimate excitation patterns with higher spatiotemporal resolution. DEAP Mapping has a potential to derive detailed changes in membrane potential from intra-operative catheter electrode signals, offering enhanced visualisation of the AF substrate from the estimated membrane potentials.
Textile-based conformable and breathable ultrasound imaging probe
Nov 07, 2023Abstract:Daily monitoring of internal tissues with conformable and breathable ultrasound (US) imaging probes is promising for early detection of diseases. In recent years, textile substrates are widely used for wearable devices since they satisfy both conformability and breathability. However, it is not currently possible to use textile substrates for US probes due to the reflection or attenuation of US waves at the air gaps in the textiles. In this paper, we fabricated a conformable and breathable US imaging probe by sandwiching the US elements between two woven polyester textiles on which copper electrodes were formed through electroless plating. The air gaps between the fibers at the electrode parts were filled with copper, allowing for high penetration of US waves. On the other hand, the non-electrode parts retain air gaps, leading to high breathability. The fabricated textile-based probe showed low flexural rigidity ($0.066 \times 10^{-4} N \cdot m^2/m$) and high air permeability ($11.7 cm^3 / cm^2 \cdot s$). Human neck imaging demonstrated the ability of the probe to monitor the pulsation of the common carotid artery and change in the internal jugular vein diameter, which lead to the early detection of health issues such as arteriosclerosis and dehydration.
Blind Signal Separation for Fast Ultrasound Computed Tomography
Apr 27, 2023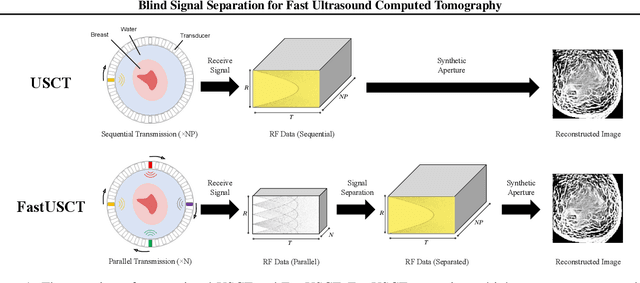
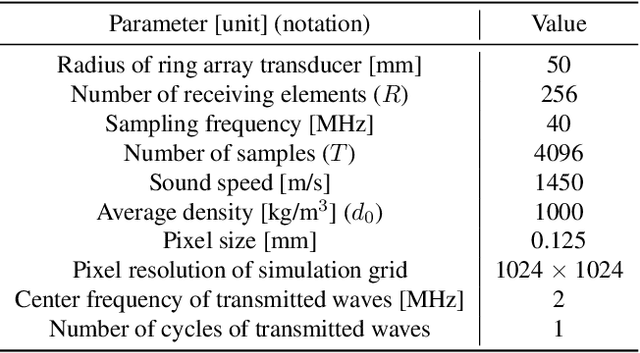
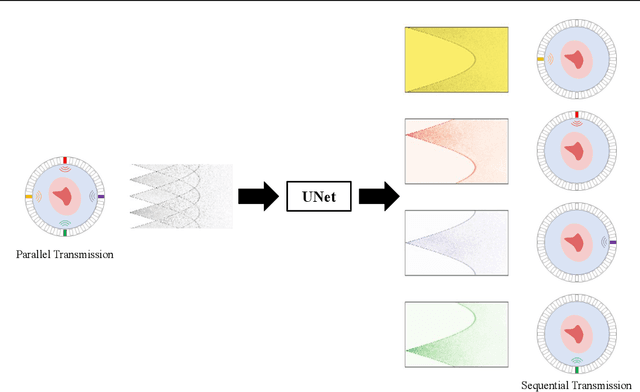
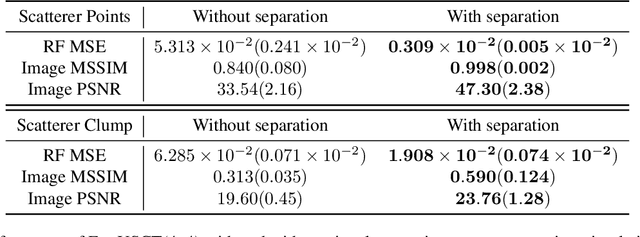
Abstract:Breast cancer is the most prevalent cancer with a high mortality rate in women over the age of 40. Many studies have shown that the detection of cancer at earlier stages significantly reduces patients' mortality and morbidity rages. Ultrasound computer tomography (USCT) is considered as a promising screening tool for diagnosing early-stage breast cancer as it is cost-effective and produces 3D images without radiation exposure. However, USCT is not a popular choice mainly due to its prolonged imaging time. USCT is time-consuming because it needs to transmit a number of ultrasound waves and record them one by one to acquire a high-quality image. We propose FastUSCT, a method to acquire a high-quality image faster than traditional methods for USCT. FastUSCT consists of three steps. First, it transmits multiple ultrasound waves at the same time to reduce the imaging time. Second, it separates the overlapping waves recorded by the receiving elements into each wave with UNet. Finally, it reconstructs an ultrasound image with a synthetic aperture method using the separated waves. We evaluated FastUSCT on simulation on breast digital phantoms. We trained the UNet on simulation using natural images and transferred the model for the breast digital phantoms. The empirical result shows that FastUSCT significantly improves the quality of the image under the same imaging time to the conventional USCT method, especially when the imaging time is limited.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge